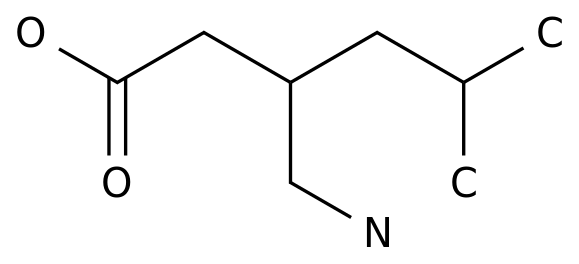
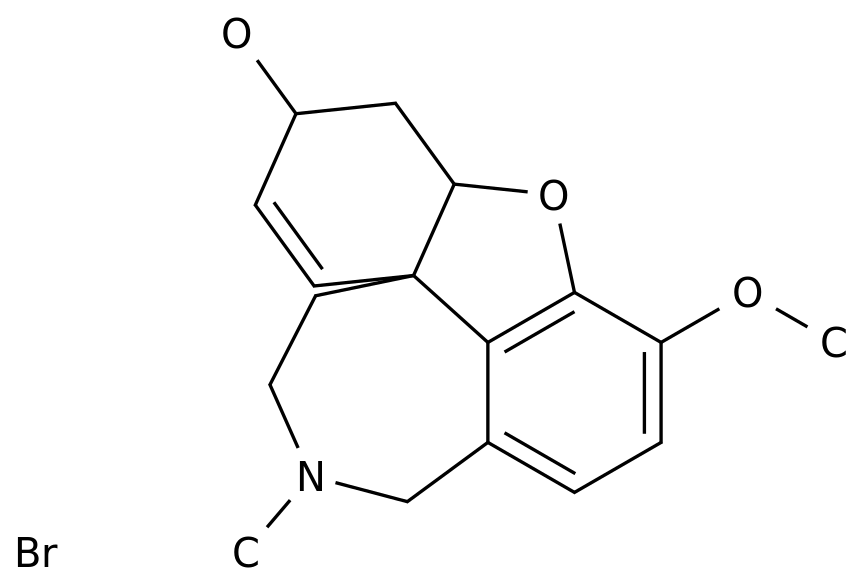
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
Written | November Responsible Editor | Mutations in Qi mutant Isocitrate dehydrogenase 1 (IDH1) can cause a molecularly specific diffuse glioma [1-3].
The most common mutation in IDH1 that causes glioma will result in a mutation in IDH1 arginine (R132H), which contains a new shared epitope that appears in the Major histocompatibility complex (MHC) of type II 【4】.
In addition to the previous research and development, a vaccine with a specific epitope of IDH1 (R132H) is called IDH1-vac.
This vaccine can induce specific and therapeutic helper T cell responses in humanized MHC mice to effectively inhibit IDH1.
(R132H) The effect of positive glioma [4].
Recently, the Michael Platten research group from the German Cancer Research Center published an article titled A vaccine targeting mutant IDH1 in newly diagnosed glioma in the journal Nature.
This is the first multi-center, single-arm, open-label study in glioma patients.
The results of phase I clinical trials have been comprehensively reported, providing a basis for future vaccine trials that target the restricted cloning of major histocompatibility complexes and individualized new epitopes in cancer immunotherapy.
In order to test the effectiveness of the vaccine, the authors first screened patients from the German Cancer Consortium (DKTK) or the neuro-oncology working group of the German Cancer Society.
33 patients were selected from 44 patients.
Suitable for entering clinical trials.
One of the patients had not been vaccinated due to fever of unknown reason.
Therefore, the total number of samples in this clinical trial was 32.These patients are divided into three categories according to the treatment plan they have experienced before registration: those who have received only radiotherapy; those who have received three rounds of temozolomide (TMZ) chemotherapy; those who have received radiotherapy and a combination of temozolomide chemotherapy.
Among them, 71.
9% of patients had received a combination of radiotherapy and chemotherapy.
According to the classification of astrocytoma by the World Health Organization, 21 out of 32 patients belonged to grade 3 astrocytoma and 11 belonged to grade 4 astrocytoma.
After categorizing the patients in detail, the authors gave each patient 8 vaccinations.
The 8 vaccines include a total of 300 ± 30μg of peptides, while ensuring that they are sterile and endotoxin-free.
One patient had a treatment-related serious adverse event, and one patient temporarily stopped the study drug due to a treatment-related adverse event.
Figure 1.
Disease progression and intervention measures of 32 patients after vaccination.
The authors made statistics on the efficacy of IDH1-vac vaccine.
The overall response rate is about 85%.
The patient's progression-free and mortality-free rates were 0.
63 and 0.
84 respectively after 3 years of vaccination.
During the experiment, 12 out of 32 patients developed pseudoprogression, that is, the phenomenon that the original lesions increased or new enhanced lesions appeared after treatment.
Through brain imaging diagnosis, it is found that this phenomenon is mainly related to the inflammatory reaction in the brain tumor.
However, the pseudo-progression of tumors mainly appears in patients with transient or sustained T cell immune responses after vaccine injection, but it does not appear in patients who do not respond.
Through in-depth T cell receptor sequencing, the authors found that specific T cell receptors appear in patients with false progress, which are the potential biological mechanisms of vaccine-induced system and local immune responses and vaccine-induced false progress Provides important insights that are related to the beneficial clinical process of some patients.
In general, this work proved the safety and immunogenicity of IDH1-vac vaccine in patients with grade 3 and 4 IDH1 (R132H) positive astrocytomas, and there were no further positive prognostic factors, reaching the level of clinical trials.
Expected end.
The experimental strategy of the IDH1-vac vaccine will provide new insights into the development of treatments for other diseases similar to glial tumors, which are particularly prone to subclonal mutations.
Original link: https://doi.
org/10.
1038/s41586-021-03363-z Platemaker: 11 References 1.
Parsons, DW et al.
An integrated genomic analysis of human glioblastoma multiforme.
Science (New York, NY ) 321, 1807-1812, doi:10.
1126/science.
1164382 (2008).
2.
Yan, H.
et al.
IDH1 and IDH2 mutations in gliomas.
The New England journal of medicine 360, 765-773, doi:10.
1056/ NEJMoa0808710 (2009) 3.
Waitkus, MS, Diplas, BH & Yan, H.
Biological Role and Therapeutic Potential of IDH Mutations in Cancer.
Cancer cell 34, 186-195, doi:10.
1016/j.
ccell.
2018.
04.
011 ( 2018).
4.
Schumacher, T.
et al.
A vaccine targeting mutant IDH1 induces antitumour immunity.
Nature 512, 324-327, doi:10.
1038/nature13387 (2014).
Reprint instructions [Original article] BioArt original article, personal repost Sharing, reprinting without permission is prohibited, the copyright of all published works is owned by BioArt.
BioArt reserves all statutory rights and offenders must be investigated.
The most common mutation in IDH1 that causes glioma will result in a mutation in IDH1 arginine (R132H), which contains a new shared epitope that appears in the Major histocompatibility complex (MHC) of type II 【4】.
In addition to the previous research and development, a vaccine with a specific epitope of IDH1 (R132H) is called IDH1-vac.
This vaccine can induce specific and therapeutic helper T cell responses in humanized MHC mice to effectively inhibit IDH1.
(R132H) The effect of positive glioma [4].
Recently, the Michael Platten research group from the German Cancer Research Center published an article titled A vaccine targeting mutant IDH1 in newly diagnosed glioma in the journal Nature.
This is the first multi-center, single-arm, open-label study in glioma patients.
The results of phase I clinical trials have been comprehensively reported, providing a basis for future vaccine trials that target the restricted cloning of major histocompatibility complexes and individualized new epitopes in cancer immunotherapy.
In order to test the effectiveness of the vaccine, the authors first screened patients from the German Cancer Consortium (DKTK) or the neuro-oncology working group of the German Cancer Society.
33 patients were selected from 44 patients.
Suitable for entering clinical trials.
One of the patients had not been vaccinated due to fever of unknown reason.
Therefore, the total number of samples in this clinical trial was 32.These patients are divided into three categories according to the treatment plan they have experienced before registration: those who have received only radiotherapy; those who have received three rounds of temozolomide (TMZ) chemotherapy; those who have received radiotherapy and a combination of temozolomide chemotherapy.
Among them, 71.
9% of patients had received a combination of radiotherapy and chemotherapy.
According to the classification of astrocytoma by the World Health Organization, 21 out of 32 patients belonged to grade 3 astrocytoma and 11 belonged to grade 4 astrocytoma.
After categorizing the patients in detail, the authors gave each patient 8 vaccinations.
The 8 vaccines include a total of 300 ± 30μg of peptides, while ensuring that they are sterile and endotoxin-free.
One patient had a treatment-related serious adverse event, and one patient temporarily stopped the study drug due to a treatment-related adverse event.
Figure 1.
Disease progression and intervention measures of 32 patients after vaccination.
The authors made statistics on the efficacy of IDH1-vac vaccine.
The overall response rate is about 85%.
The patient's progression-free and mortality-free rates were 0.
63 and 0.
84 respectively after 3 years of vaccination.
During the experiment, 12 out of 32 patients developed pseudoprogression, that is, the phenomenon that the original lesions increased or new enhanced lesions appeared after treatment.
Through brain imaging diagnosis, it is found that this phenomenon is mainly related to the inflammatory reaction in the brain tumor.
However, the pseudo-progression of tumors mainly appears in patients with transient or sustained T cell immune responses after vaccine injection, but it does not appear in patients who do not respond.
Through in-depth T cell receptor sequencing, the authors found that specific T cell receptors appear in patients with false progress, which are the potential biological mechanisms of vaccine-induced system and local immune responses and vaccine-induced false progress Provides important insights that are related to the beneficial clinical process of some patients.
In general, this work proved the safety and immunogenicity of IDH1-vac vaccine in patients with grade 3 and 4 IDH1 (R132H) positive astrocytomas, and there were no further positive prognostic factors, reaching the level of clinical trials.
Expected end.
The experimental strategy of the IDH1-vac vaccine will provide new insights into the development of treatments for other diseases similar to glial tumors, which are particularly prone to subclonal mutations.
Original link: https://doi.
org/10.
1038/s41586-021-03363-z Platemaker: 11 References 1.
Parsons, DW et al.
An integrated genomic analysis of human glioblastoma multiforme.
Science (New York, NY ) 321, 1807-1812, doi:10.
1126/science.
1164382 (2008).
2.
Yan, H.
et al.
IDH1 and IDH2 mutations in gliomas.
The New England journal of medicine 360, 765-773, doi:10.
1056/ NEJMoa0808710 (2009) 3.
Waitkus, MS, Diplas, BH & Yan, H.
Biological Role and Therapeutic Potential of IDH Mutations in Cancer.
Cancer cell 34, 186-195, doi:10.
1016/j.
ccell.
2018.
04.
011 ( 2018).
4.
Schumacher, T.
et al.
A vaccine targeting mutant IDH1 induces antitumour immunity.
Nature 512, 324-327, doi:10.
1038/nature13387 (2014).
Reprint instructions [Original article] BioArt original article, personal repost Sharing, reprinting without permission is prohibited, the copyright of all published works is owned by BioArt.
BioArt reserves all statutory rights and offenders must be investigated.