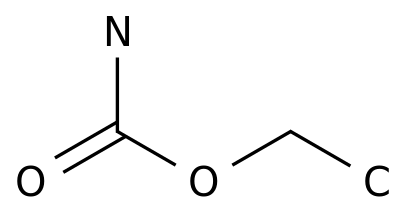
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
Responsible Editor | The normal function of the central nervous system is a combination of excitatory neurons and inhibitory neurons.
GABA and glycine are the main neurotransmitters that conduct inhibitory signals, while glutamate is the main neurotransmitter that conducts excitatory signals.
These neurotransmitter-bound receptors are highly concentrated on the postsynaptic membrane and mediate nerve signal transduction in the brain and spinal cord.
AMPAR (AMPA-selective glutamate receptor) and other glutamate receptors are the main mediators of excitatory signals in the nervous system.
AMPAR is a cation channel regulated by a ligand.
When the presynaptic membrane releases glutamate, AMPAR is activated by glutamate to open ion channels.
Sodium and calcium ions enter the inner cell membrane, causing depolarization of the cell membrane, thereby transmitting excitatory signals.
The hippocampus is an important part of the brains of humans and vertebrates.
It plays a role in short-term memory, long-term memory, and spatial positioning.
Many neurodegenerative diseases are related to the hippocampus, such as Alzheimer's disease.
Long-term studies have proved that AMPAR plays a very key role in the signal transmission of neural circuits in the hippocampus.
AMPAR is a tetramer composed of four subtypes GluA1, GluA2, GluA3 and GluA4.
It is composed of N-terminal region (ATD), ligand binding region (LBD) and transmembrane region (TMD).
Ion channels are located in the transmembrane zone and allow cations to pass through.
AMPAR does not exist alone in the body.
It is surrounded by many auxiliary subunits, regulating the function, transportation and expression of AMPAR.
The composition of AMPAR is different in different brain regions.
Studies have shown that GluA1 and GluA2 are the most abundant subunits in the hippocampus, while TARP-γ8, CNIH2 and SynDIG4 are the main auxiliary proteins of AMPAR in the hippocampus.
In order to study which subtypes of AMPAR complexes are expressed in the hippocampus, which accessory proteins are commonly assembled with AMPAR, and how accessory protein-specific antagonists inhibit AMPAR in its natural state, May 12, 2021, Eric, Oregon Health Science University, USA Yu Jie and others of the Gouaux group published a research paper entitled Hippocampal AMPA receptor assemblies and mechanism of allosteric inhibition in Nature.
The research team used single-molecule fluorescence imaging technology, electrophysiological experiments and cryo-electron microscopy to reveal for the first time the composition of the AMPAR complex in the hippocampus, the composition of accessory proteins, and the inhibitory mechanism of specific antagonists.
The research team dissected the brains of 200 mice, and extracted the natural AMPAR complex in the hippocampus using GluA2 specific monoclonal antibodies from 400 hippocampus.
The research team collected a large number of cryo-electron microscope receipts, and through computational analysis, revealed that there are four main complexes in the hippocampus, of which GluA1 and GluA2 are the most important subunits of the complex.
At the same time, this conclusion is proved by single-molecule fluorescence imaging technology.
The research team obtained a high-resolution GluA1-GluA2-TARP-γ8-CNIH2 complex structure by separating and focusing on the LBD-TMD region, and found that TARP-γ8 and CNIH2 are located at B'/D' and A'/ respectively.
C'position, and analyzed how TARP-γ8 and CNIH2 interact with AMPAR to regulate the function of AMPAR (Figure 1).
Figure 1: Structure and composition of the complex of GluA1-GluA2-TARP-γ8-CNIH2 JNJ is a non-competitive antagonist of AMPAR, which specifically acts on AMPAR containing TRAP-γ8.
Compared with classic antagonists used to treat neurological diseases (such as epilepsy), it is believed to have the lowest side effects and cause the least movement disorders.
The research team analyzed the structure of the hippocampal AMPAR complex in the presence of JNJ, and through a large number of electrophysiological experiments, revealed how JNJ binds to the TARP-γ8-AMPAR interface and makes AMPAR in the off state of inhibition mechanism.
This proves the feasibility of using the natural neurotransmitter receptor as a drug target to design small molecules.
Finally, the research team also found that the third auxiliary subunit SynDIG4 is also present in the hippocampal AMPAR complex, and predicted its possible mechanism for regulating AMPAR function (Figure 2).
Figure 2: A cartoon schematic diagram of the hippocampus AMPAR complex.
Original link: Reprinting instructions [Non-original article] The copyright of this article belongs to the author of the article, and individuals are welcome Reposting and sharing, reprinting without permission is prohibited, the author has all legal rights, offenders must be investigated.
GABA and glycine are the main neurotransmitters that conduct inhibitory signals, while glutamate is the main neurotransmitter that conducts excitatory signals.
These neurotransmitter-bound receptors are highly concentrated on the postsynaptic membrane and mediate nerve signal transduction in the brain and spinal cord.
AMPAR (AMPA-selective glutamate receptor) and other glutamate receptors are the main mediators of excitatory signals in the nervous system.
AMPAR is a cation channel regulated by a ligand.
When the presynaptic membrane releases glutamate, AMPAR is activated by glutamate to open ion channels.
Sodium and calcium ions enter the inner cell membrane, causing depolarization of the cell membrane, thereby transmitting excitatory signals.
The hippocampus is an important part of the brains of humans and vertebrates.
It plays a role in short-term memory, long-term memory, and spatial positioning.
Many neurodegenerative diseases are related to the hippocampus, such as Alzheimer's disease.
Long-term studies have proved that AMPAR plays a very key role in the signal transmission of neural circuits in the hippocampus.
AMPAR is a tetramer composed of four subtypes GluA1, GluA2, GluA3 and GluA4.
It is composed of N-terminal region (ATD), ligand binding region (LBD) and transmembrane region (TMD).
Ion channels are located in the transmembrane zone and allow cations to pass through.
AMPAR does not exist alone in the body.
It is surrounded by many auxiliary subunits, regulating the function, transportation and expression of AMPAR.
The composition of AMPAR is different in different brain regions.
Studies have shown that GluA1 and GluA2 are the most abundant subunits in the hippocampus, while TARP-γ8, CNIH2 and SynDIG4 are the main auxiliary proteins of AMPAR in the hippocampus.
In order to study which subtypes of AMPAR complexes are expressed in the hippocampus, which accessory proteins are commonly assembled with AMPAR, and how accessory protein-specific antagonists inhibit AMPAR in its natural state, May 12, 2021, Eric, Oregon Health Science University, USA Yu Jie and others of the Gouaux group published a research paper entitled Hippocampal AMPA receptor assemblies and mechanism of allosteric inhibition in Nature.
The research team used single-molecule fluorescence imaging technology, electrophysiological experiments and cryo-electron microscopy to reveal for the first time the composition of the AMPAR complex in the hippocampus, the composition of accessory proteins, and the inhibitory mechanism of specific antagonists.
The research team dissected the brains of 200 mice, and extracted the natural AMPAR complex in the hippocampus using GluA2 specific monoclonal antibodies from 400 hippocampus.
The research team collected a large number of cryo-electron microscope receipts, and through computational analysis, revealed that there are four main complexes in the hippocampus, of which GluA1 and GluA2 are the most important subunits of the complex.
At the same time, this conclusion is proved by single-molecule fluorescence imaging technology.
The research team obtained a high-resolution GluA1-GluA2-TARP-γ8-CNIH2 complex structure by separating and focusing on the LBD-TMD region, and found that TARP-γ8 and CNIH2 are located at B'/D' and A'/ respectively.
C'position, and analyzed how TARP-γ8 and CNIH2 interact with AMPAR to regulate the function of AMPAR (Figure 1).
Figure 1: Structure and composition of the complex of GluA1-GluA2-TARP-γ8-CNIH2 JNJ is a non-competitive antagonist of AMPAR, which specifically acts on AMPAR containing TRAP-γ8.
Compared with classic antagonists used to treat neurological diseases (such as epilepsy), it is believed to have the lowest side effects and cause the least movement disorders.
The research team analyzed the structure of the hippocampal AMPAR complex in the presence of JNJ, and through a large number of electrophysiological experiments, revealed how JNJ binds to the TARP-γ8-AMPAR interface and makes AMPAR in the off state of inhibition mechanism.
This proves the feasibility of using the natural neurotransmitter receptor as a drug target to design small molecules.
Finally, the research team also found that the third auxiliary subunit SynDIG4 is also present in the hippocampal AMPAR complex, and predicted its possible mechanism for regulating AMPAR function (Figure 2).
Figure 2: A cartoon schematic diagram of the hippocampus AMPAR complex.
Original link: Reprinting instructions [Non-original article] The copyright of this article belongs to the author of the article, and individuals are welcome Reposting and sharing, reprinting without permission is prohibited, the author has all legal rights, offenders must be investigated.