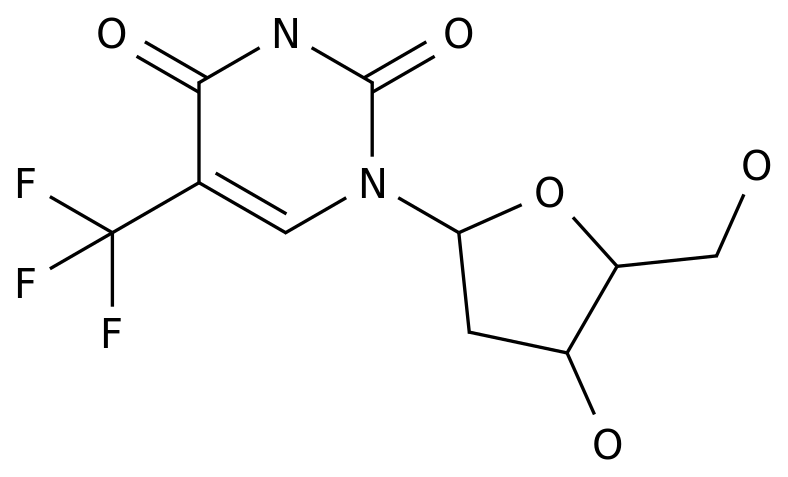
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
On June 5, 1981, the US Centers for Disease Control and Prevention published a case report of 5 AIDS patients, which was the first official record of AIDS in the world
.
Mankind has been committed to conquering AIDS for 40 years.
During this period, two cases of AIDS cure were well-known to everyone.
All this seems to indicate that AIDS will one day be missed from the title of "incurable disease"
.
To end AIDS, prevention is one of the focuses of scientists
.
Recently, a research team from Oregon Health and Science University in the United States published an article titled Antibody-based CCR5 blockade protects Macaques from mucosal SHIV transmission in the Nautre journal
.
Its research shows that the humanized CCR5 monoclonal antibody Leronlimab can effectively prevent non-primates such as rhesus monkeys from infecting ape/human HIV virus.
This research result will provide a new clinical treatment approach for humans to prevent HIV infection
.
https://doi.
org/10.
1038/s41467-021-23697-6 In the current situation where HIV prevention vaccines have not been successfully developed, antiretroviral therapy (ART) is the main pre-exposure preventive medication ( Pre-exposure prophylaxis (PrEP) is a means to slow down the AIDS epidemic.
However, due to low drug adherence and the rising rate of drug resistance, ART therapy seems to have reached a deadlock
.
In the two previously reported cases of AIDS cure, scientists found that individuals carrying CCR5-Δ32 mutations can significantly resist HIV infection.
Therefore, CCR5 is an ideal target for preventing HIV infection.
This research is also centered on targeting CCR5.
Leronlimab launched an investigation to explore whether it can be an effective PrEP formulation
.
In this study, the researchers first evaluated the ability of Leronlimab to inhibit the spread of HIV virus between cells in an in vitro diffusion test.
They concluded that in a pure and highly activated CD4+ T cell in vitro culture system, 100 μg/mL Leronlimab can completely resist the combination of CCR5 receptor and HIV virus, thereby preventing the spread of the virus
.
The test results confirmed this point.
When the same concentration of Leronlimab was used for the diffusion test, CD4+ T cells from wild-type CCR5 donors were able to completely resist HIV infection, and their preventive ability was equivalent to that of those carrying the CCR5-Δ32 mutation.
.
After testing with 25 isolated HIV strains, the researchers found that Leronlimab can mimic the effect of CCR5 Δ32/Δ32 on CD4+ T cells and completely resist HIV infection
.
The test results showed that Leronlimab blocked the spread of CCR5 in vitro.
Subsequently, the research team evaluated whether Leronlimab could be used as PrEP to withstand repeated low-dose intrarectal (intrarectal, IR) SIV virus invasion.
They used rhesus monkeys as a victim.
The trial was divided into 3 groups, each with 6 animals.
Group 1 was a control group without any treatment or prevention, group 2 was a test group treated with Leronlimab at a dose of 10 mg/kg per week, and group 3 was a control group that was treated with Leronlimab every week.
In the experimental group treated with Leronlimab at a dose of 50 mg/kg weekly, Group 2 and Group 3 represent the lowest and highest doses used in clinical trials of Leronlimab
.
Each group will undergo IR testing for 8 consecutive weeks.
Infected subjects will be euthanized, and uninfected subjects will be monitored for a long time
.
The test results showed that all animals showed good tolerance to Leronlimab without adverse clinical reactions, which confirmed its high safety reported in multiple previous clinical trials
.
At the same time, all the untreated rhesus monkeys in group 1 were infected, two rhesus monkeys in group 2 were infected, and all subjects in group 3 remained virus-free, which indicated that the use of Leronlimab allowed all rhesus monkeys in group 3 Rhesus monkeys have the ability to prevent HIV infection and also show its feasibility as PrEP in preventing HIV infection
.
Leronlimab is used as the PrEP test procedure and results.
At the end, it is mentioned that Leronlimab can be administered subcutaneously at home, which is more advantageous than other intramuscular injections that need to be administered in the clinic
.
At the same time, compared with rhesus monkeys, human Leronlimab has a significantly longer plasma half-life, coupled with the lower expression of CCR5 on human CD4+ T cells, so monthly Leronlimab may be sufficient to prevent HIV infection
.
With the advancement of antibody engineering technology to increase the plasma half-life, Leronlimab may be extended to a quarterly injection, thereby further improving drug compliance by reducing the frequency of dosing
.
In view of the fact that the CCR5 Δ32/Δ32 phenotype can resist HIV virus infection on the basis of no threat to life, and the safety of Leronlimab has also been further verified.
In the future, it is necessary for scientists to treat and prevent Leronlimab in AIDS.
Do more in-depth research
.
End reference materials: [1]https://
.
Mankind has been committed to conquering AIDS for 40 years.
During this period, two cases of AIDS cure were well-known to everyone.
All this seems to indicate that AIDS will one day be missed from the title of "incurable disease"
.
To end AIDS, prevention is one of the focuses of scientists
.
Recently, a research team from Oregon Health and Science University in the United States published an article titled Antibody-based CCR5 blockade protects Macaques from mucosal SHIV transmission in the Nautre journal
.
Its research shows that the humanized CCR5 monoclonal antibody Leronlimab can effectively prevent non-primates such as rhesus monkeys from infecting ape/human HIV virus.
This research result will provide a new clinical treatment approach for humans to prevent HIV infection
.
https://doi.
org/10.
1038/s41467-021-23697-6 In the current situation where HIV prevention vaccines have not been successfully developed, antiretroviral therapy (ART) is the main pre-exposure preventive medication ( Pre-exposure prophylaxis (PrEP) is a means to slow down the AIDS epidemic.
However, due to low drug adherence and the rising rate of drug resistance, ART therapy seems to have reached a deadlock
.
In the two previously reported cases of AIDS cure, scientists found that individuals carrying CCR5-Δ32 mutations can significantly resist HIV infection.
Therefore, CCR5 is an ideal target for preventing HIV infection.
This research is also centered on targeting CCR5.
Leronlimab launched an investigation to explore whether it can be an effective PrEP formulation
.
In this study, the researchers first evaluated the ability of Leronlimab to inhibit the spread of HIV virus between cells in an in vitro diffusion test.
They concluded that in a pure and highly activated CD4+ T cell in vitro culture system, 100 μg/mL Leronlimab can completely resist the combination of CCR5 receptor and HIV virus, thereby preventing the spread of the virus
.
The test results confirmed this point.
When the same concentration of Leronlimab was used for the diffusion test, CD4+ T cells from wild-type CCR5 donors were able to completely resist HIV infection, and their preventive ability was equivalent to that of those carrying the CCR5-Δ32 mutation.
.
After testing with 25 isolated HIV strains, the researchers found that Leronlimab can mimic the effect of CCR5 Δ32/Δ32 on CD4+ T cells and completely resist HIV infection
.
The test results showed that Leronlimab blocked the spread of CCR5 in vitro.
Subsequently, the research team evaluated whether Leronlimab could be used as PrEP to withstand repeated low-dose intrarectal (intrarectal, IR) SIV virus invasion.
They used rhesus monkeys as a victim.
The trial was divided into 3 groups, each with 6 animals.
Group 1 was a control group without any treatment or prevention, group 2 was a test group treated with Leronlimab at a dose of 10 mg/kg per week, and group 3 was a control group that was treated with Leronlimab every week.
In the experimental group treated with Leronlimab at a dose of 50 mg/kg weekly, Group 2 and Group 3 represent the lowest and highest doses used in clinical trials of Leronlimab
.
Each group will undergo IR testing for 8 consecutive weeks.
Infected subjects will be euthanized, and uninfected subjects will be monitored for a long time
.
The test results showed that all animals showed good tolerance to Leronlimab without adverse clinical reactions, which confirmed its high safety reported in multiple previous clinical trials
.
At the same time, all the untreated rhesus monkeys in group 1 were infected, two rhesus monkeys in group 2 were infected, and all subjects in group 3 remained virus-free, which indicated that the use of Leronlimab allowed all rhesus monkeys in group 3 Rhesus monkeys have the ability to prevent HIV infection and also show its feasibility as PrEP in preventing HIV infection
.
Leronlimab is used as the PrEP test procedure and results.
At the end, it is mentioned that Leronlimab can be administered subcutaneously at home, which is more advantageous than other intramuscular injections that need to be administered in the clinic
.
At the same time, compared with rhesus monkeys, human Leronlimab has a significantly longer plasma half-life, coupled with the lower expression of CCR5 on human CD4+ T cells, so monthly Leronlimab may be sufficient to prevent HIV infection
.
With the advancement of antibody engineering technology to increase the plasma half-life, Leronlimab may be extended to a quarterly injection, thereby further improving drug compliance by reducing the frequency of dosing
.
In view of the fact that the CCR5 Δ32/Δ32 phenotype can resist HIV virus infection on the basis of no threat to life, and the safety of Leronlimab has also been further verified.
In the future, it is necessary for scientists to treat and prevent Leronlimab in AIDS.
Do more in-depth research
.
End reference materials: [1]https://