-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
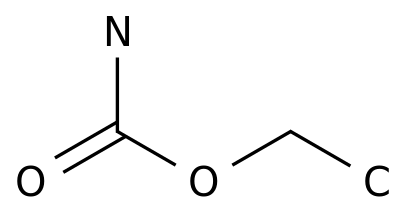
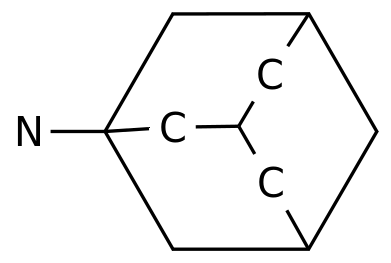
September 24, 2020 // -- Eisai (Eisai) recently announced that the European Medicines Agency (EMA) Committee on Human Pharmaceutical Products (CHMP) has issued a positive review of the approval of the anti-epileptic drug Fycompa (Wycompa®, general name: perampanel, lun panai) to expand the applicable population: (1) ) As an auxiliary therapy to treat partial seizure epilepsy (POS, with or without or without secondary systemic seizures), the age range was expanded from 12 years of age and above to 4 years of age and above;
application to expand the paediatric patient population through Fycompa was submitted to the EMA in February 2019.
application is based on data from Phase III Clinical Studies (Study 311) and Phase II Clinical Studies (Study 232), which evaluated Fycompa as an ad lid therapy for pediatric epilepsy patients.
The Study 311 study assessed the relationship between the safety, tolerance, efficacy and blood drug concentration of pediatric patients (4 to 12 years of age) who were not fully controlled for partial or severe spasms treated as an auxiliary therapy.
Study 232 study evaluated Fycompa's pharmacodynamics, efficacy, and long-term safety as an ad litherapy treatment for pediatric patients (under 2 to 12 years of age).
Fycompa is a pioneering (first-in-class) anti-epileptic drug (AEDs) developed in-house, a highly selective, non-competitive AMPA glutamate-type antagonist.
glutamate is the main neurotransmitter that mediates seizures.
As an AMPA-like antagonist, Fycompa reduces excessive excitation of neurons associated with seizures by targeting the activity of the AMPA-Glutamate after synapses;
To date, Fycompa has been approved by more than 70 countries worldwide, including Japan, the United States, China, and other countries in Europe and Asia, as an ancillary therapy for the treatment of partial seizures (POS, with or without secondary systemic seizures) in patients aged 12 and over.
In addition, Fycompa has been approved by more than 65 countries around the world, including the United States, Japan, Europe and Asia, as an ad liable therapy for the treatment of primary full-range PGTC seizures in patients 12 years of age and older with epilepsy.
in the United States and Japan, Fycompa is also suitable as a single drug therapy and complementary therapy for the treatment of partial seizures (with or without secondary systemic seizures) in patients 4 years of age and older.
, Fycompa has been used to treat more than 300,000 patients worldwide.
currently, Wesser is also conducting a global Phase III clinical study (Study 338) to evaluate Fycompa's treatment for epilepsy associated with Lennox-Gastaut syndrome.
addition, the company is also developing Fycompa an injection preparation.
in China, Fycompa (Wyketai ®, common name: perampanel, ville panai) submitted a new drug application (NDA) in September 2018 to treat partial seizures in patients 12 years of age and older with epilepsy.
China's State Drug Administration (NMPA) granted Fycompa priority review eligibility in January 2019 and Fycompa in September 2019 because of its significant clinical benefits with existing drugs.
In early January this year, Wesser introduced Fycompa (Wycombe ®), a daily tablet for complementary treatment of partial seizure epilepsy (accompanied or not accompanied by secondary systemic epilepsy) in patients aged 12 and over.
estimates, there are about 9 million people with epilepsy in China, about 60% of whom are affected by partial seizures, of which 40% need complementary treatment.
about 30% of people with epilepsy receive marketable anti-epileptic drugs (AEDs) that do not control seizures, there are significant unsealed medical needs in this area.
epilepsy can be roughly classified according to the type of seizures, of which partial seizures account for about 60% of seizure cases, systemic seizures account for about 40%.
primary all-round strong straightness (PGTC) seizures, or grand mal, are the most common and severe types of systemic seizures, accounting for about 60% of systemic seizures.
PGTC seizures to loss of consciousness and systemic convulsions as the characteristics of common epileptic seizures are mainly spitting white foam, two eyes turned over, limbs convulsions, screaming and other serious causes of urination incontinence, sustained seizures and so on.
seizures are the result of imbalances in brain neurons that can be triggered by a variety of neurochemical mechanisms, but little is known.
() Origin: Eisai Receives Positive Opinion From EMA's CHMP On Use Of Antiepileptic Agent Fycompa In Pediatric Patients.