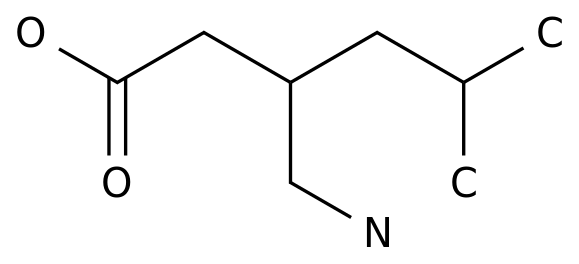
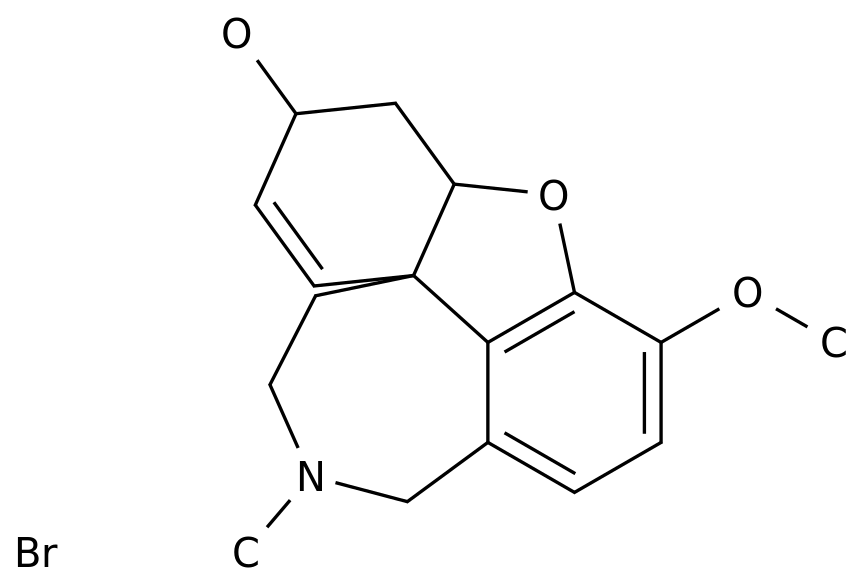
New drugs for schizophrenia! Captrita (lumateprone), an ICT company, has shown a strong therapeutic effect in the treatment of patients with acute exacerbation, and will be on the market soon!
-
Last Update: 2020-02-22
-
Source: Internet
-
Author: User
Search more information of high quality chemicals, good prices and reliable suppliers, visit
www.echemi.com
February 22, 2020 / BIOON / -- intra cellular therapeutics (ICT) is a biopharmaceutical company focusing on developing innovative therapies for central nervous system (CNS) diseases Recently, the company announced that the results of the phase III clinical trial (iti-007-301) to evaluate the treatment of adults with schizophrenia by capleta (lumateprone) have been published in the Journal of the American Medical Association psychiatry The title of the article is: efficient and safety of lumateprone for treatment of schizophrenia: a random clinical trial Caplyta was approved by the U.S Food and Drug Administration (FDA) in December 2019 for the treatment of adults with schizophrenia In terms of medication, the recommended dose of caplyta is 42mg, once a day, taken with food, and dose titration is not required It should be noted that the drug label of caplyta contains a black box warning, which indicates that the risk of death of patients with dementia related psychosis is increased due to the use of antipsychotics; and that caplyta is not approved for the treatment of dementia related psychosis ICT has previously said it will bring caplyta to market by the end of the first quarter of 2020 Iti-007-301 is a randomized, double-blind, fixed dose, placebo-controlled phase III clinical trial conducted in 12 clinical centers in the United States A total of 450 patients were enrolled These patients were diagnosed as schizophrenia by dsm-5 standard and had acute aggravation of psychiatric symptoms In the study, patients were randomly (1:1:1) assigned to receive caplyta 42mg, 28mg, placebo, once a day in the morning, for 4 weeks The primary pre-determined efficacy evaluation was the change in the total score of the central assessment positive and negative symptom scale (PANSS) from baseline to the end of the study (week 4) The key secondary end point was the central assessment of the CGI-S score The mean baseline PANSS score of the patients in the study was 89.8, indicating significant morbidity The 28mg dose in the study was not an approved dose for caplyta The results showed that the dose of caplyta 42mg reached the primary end point, and the total PANSS score of the 4th week was compared with the baseline, showing the antipsychotic effect, with a statistically significant advantage compared with placebo (drug placebo difference: - 4.2 points) In addition, caplyta 42mg also reached a key secondary endpoint for statistically significant improvement in CGI-S scores The 42mg dose of caplyta showed a significant antipsychotic effect as early as week 1, and maintained this effect at every time point throughout the study In the study, the most common adverse events in the caplyta 42mg group were lethargy (17.3% vs 4.0%), sedation (12.7% vs 5.4%), fatigue (5.3% vs 1.3%), and constipation (6.7% vs 2.7%) Compared with placebo, there was no significant difference in body weight, cholesterol, triglyceride, glucose, insulin, prolactin and other metabolic parameters In any treatment group, the incidence of adverse events (teaes) associated with extrapyramidal symptoms (EPS) was less than 5% Dr Christoph Correll, Professor of psychiatry and molecular medicine, Hofstra / northwell Zuck School of medicine, New York, said: "the 42mg dose of caplyta significantly improved the symptoms of patients with acute exacerbation of schizophrenia, with good tolerance Caplyta represents an important complement to healthcare providers in dealing with this heterogeneous mental state " Schizophrenia is a serious mental disease, affecting about 2.4 million adults in the United States The clinical manifestations of schizophrenia are various Acute episodes are characterized by psychiatric symptoms, including hallucinations and delusions, often requiring hospitalization The disease is chronic and lifelong, often accompanied by depression and progressive deterioration of social function and cognitive ability Schizophrenia patients often stop treatment because of side effects such as weight gain and dyskinesia The active component of caplyta is lumateprone, which is a first-in-class small molecule drug It can selectively and simultaneously regulate 5-hydroxytryptamine, dopamine and glutamate, three neurotransmitter pathways involved in serious diseases Lumateperone is a dopamine receptor phosphoprotein modulator (DPPM), which acts as a presynaptic partial agonist and postsynaptic antagonist on D2 receptor This mechanism, together with potential interaction with 5-HT2A receptor, 5-hydroxytryptamine transporter and D1 receptor, and indirect glutamate regulation, may contribute to lumateprone's efficacy across a range of psychiatric symptoms, with improved psychosocial function and good tolerance The compound has the potential to benefit patients with a range of neuropsychiatric disorders and neurodegenerative diseases In the United States, the FDA has granted lumateprone a fast track status for schizophrenia treatment in November 2017 In addition to schizophrenia, ICT is also developing lumateprone for the treatment of other mental disorders, including behavioral disorders in dementia patients, Alzheimer's disease, depression and other neuropsychiatric and neurological disorders In July 2019, lumateprone was used as the top line result of two phase III clinical studies (study 401, study 404) in the treatment of bipolar I or bipolar II related major depression with single drug therapy Data showed that in the 404 study, lumateprone 42mg reached the primary end point (P < 0.001) and the secondary end point (P < 0.001) of improving the severity of depression compared with placebo group However, in the 401 study, due to the high clinical response of the placebo group, the two doses of lumateperone (42mg and 28mg) did not reach the primary end point Lumateperone showed good safety and tolerance in two studies (BIOON Com) original source: pivotal study of caplyta (lumateprone) for the treatment of schizophrenia in adults published in JAMA psychiatry
This article is an English version of an article which is originally in the Chinese language on echemi.com and is provided for information purposes only.
This website makes no representation or warranty of any kind, either expressed or implied, as to the accuracy, completeness ownership or reliability of
the article or any translations thereof. If you have any concerns or complaints relating to the article, please send an email, providing a detailed
description of the concern or complaint, to
service@echemi.com. A staff member will contact you within 5 working days. Once verified, infringing content
will be removed immediately.