-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
This article is original by Translational Medicine Network.
Please indicate the source for reprinting.
Author: John Guide: CStone Pharmaceuticals has further highlighted its advantages in the field of precision therapy and immunotherapy.
It announced the joint development of lorlatinib (lorlatinib, formerly known as Lao) with Pfizer.
After latinib), its pivotal study on ROS1-positive NSCLC patients was approved by the NMPA
.
Prior to this, Pfizer's loratinib has been approved by the FDA for the first-line treatment of anaplastic lymphoma kinase (ALK)-positive and treated ALK-positive metastatic non-small cell lung cancer (NSCLC) patients
.
CStone Pharmaceuticals announced on January 4 that the clinical trial application (IND) of loratinib for ROS1-positive advanced non-small cell lung cancer (NSCLC) has been approved by the National Medical Products Administration (NMPA) of China
.
This is the world's first pivotal study of loratinib in the treatment of ROS1-positive NSCLC
.
It is understood that the study aims to evaluate the anti-tumor activity and safety of loratinib in patients with ROS1-positive advanced NSCLC.
It will enroll ROS1-positive advanced NSCLC patients who have failed chemotherapy and precision treatment.
The primary endpoint of the study is independent review.
The objective response rate (ORR) assessed by the committee
.
In a previous phase I/II study, loratinib showed a deep and lasting objective remission in ROS1-positive advanced NSCLC patients who had not been treated with TKI or had failed TKI treatment
.
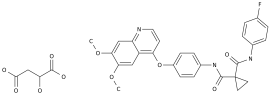
As the third-generation ALK/ROS1 tyrosine kinase inhibitor, loratinib can provide a new treatment option for patients with ROS1-positive advanced non-small cell lung cancer
.
Lolatinib is a third-generation ALK/ROS1 tyrosine kinase inhibitor (TKI) with potent and selective inhibitory activity and can penetrate the blood-brain barrier
.
With its excellent clinical data, loratinib has been approved for the treatment of adult ALK-positive metastatic NSCLC patients in more than 50 countries around the world
.
Loratinib is developed and produced by Pfizer, with diverse and powerful effects
.
According to the clinical data of lolatinib, for different types of ALK+ patients, the first-line use of lolatinib has an effective rate of up to 90%; for patients who are resistant to crizotinib, continue to use lolatinib , The effective rate can reach about 60%; for patients who are resistant to all three ALK inhibitors, the efficiency of loratinib can be as high as about 39%
.
In March of this year, the US FDA approved Lorbrena (lorlatinib) to expand its indications for the first-line treatment of patients with anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer (NSCLC)
.
Lorbrena was granted accelerated approval in the United States in 2018 for treated ALK-positive metastatic non-small cell lung cancer (NSCLC) patients
.
This approval also translates the accelerated approval in 2018 into a full approval
.
It is worth noting that this research is a further deepening of the strategic cooperation between CStone Pharmaceuticals and Pfizer
.
In September 2020, CStone Pharmaceuticals and Pfizer reached a strategic cooperation.
Pfizer will purchase 9.
90% of CStone Pharmaceuticals (valued at US$200 million), which also includes the cooperation framework for the two parties to introduce more oncology products into the Greater China region
.
In June 2021, CStone Pharmaceuticals and Pfizer jointly announced that the two parties will jointly develop loratinib in Greater China and conduct research on ROS1-positive NSCLC, which will bring more treatment options to patients in the cancer field
.
Popular·Article Basic Research [Science Sub-Journal] Transforming artificial lungs-lizards provide amazing methods! Intestinal flora [Nature] Did you take hypoglycemic drugs ineffective? Because the intestinal microflora is in the process of cancer treatment [PNAS] Chinese medicinal materials thyme and oregano have found anti-cancer compounds for disease diagnosis and treatment [Nature Sub-Journal] This is another step towards changing the US$70 billion global diagnostic industry! Protein biosensors can measure toxic drugs for cardiovascular disease in cancer, arthritis, and organ transplant patients [JAMA Sub-Journal] Are you stressed? Stress increases the risk of cardiovascular disease! Scientific research [Cell Sub-Journal] Why do pain and anxiety speed up the breathing rate? The scientific research "Science" announced the breakthrough of 2021: a problem that has troubled biologists for 50 years was solved by AI and realized the dream of a Nobel Prize winner!