-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
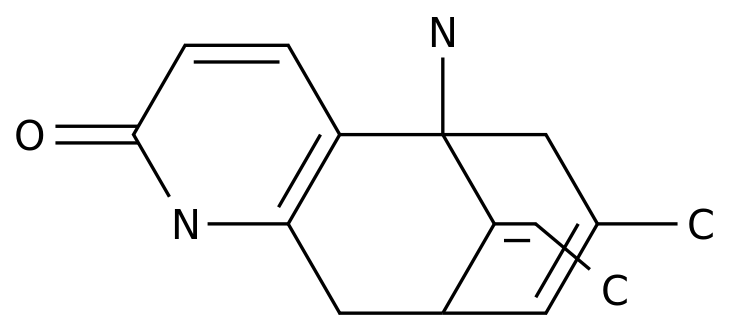
October 07, 2020 // -- Sunovion Pharmaceuticals, the U.S. subsidiary of Sumayou Pharmaceuticals of Japan, recently announced the launch of Kynmobi (apomorphine HCl, hydrochloric acid morphine) sub-tongue membrane agent in the U.S. market. (sublingual film), a drug approved by the FDA in May 2020 for acute intermittent treatment of fluctuations in motor symptoms in patients with Parkinson's disease (PD), or OFF episodes, "OFF" events.
Kynmobi is a new thin-film preparation for apomorphine, a dopamine D2-subjector astrogen used as an emergency drug for OFF events, approved in the United States in the form of subsulphage injections.
Kynmobi is an under-the-tongue drug that dissolves under the tongue and is given under a simple tongue to help PD patients improve their OFF symptoms as needed.
it's worth noting that Kynmobi is the first and only sub-tongue therapy to treat OFF events of Parkinson's disease quickly and on demand, up to five times a day.
phase III study, patients treated with Kynmobi had significantly improved their exercise symptoms within 30 minutes compared to placebos.
(PD) is a chronic neurodegenerative disease in which cells that produce dopamine are lost.
predicts that by 2030, the total number of PD patients in the United States will reach 1.2 million.
up to 60% of PD patients experience OFF events within 4-6 years of diagnosis, regardless of the severity of the disease.
OFF event is the re-emergence or exacerbation of Parkinson's disease symptoms, some of which include tremors, stiffness, slow movement, or other symptoms, in the case of oral L-Doba/Kabidoba control.
these destructive events can occur when you wake up in the morning and throughout the day.
OFFF event is a common and challenging part of Parkinson's disease, and the unforceability of its onset can be extremely challenging and disruptive to the daily lives of Parkinson's patients and their caregivers. Thomas Gibbs, chief commercial officer at
Sunovion, said: "PD patients may spend hours a day dealing with interference caused by OFF events, which can lead to reduced motor function and challenge daily activities.
OF EVENT can make simple tasks, such as wearing clothes in the morning, almost impossible.
we are pleased that PD patients and their caregivers and doctors can now use Kynmobi to control these debilitating intermittent episodes. The results of phase III clinical trials published in The Lancet Neurology by
showed that in week 12 of treatment, Parkinson's patients showed significant improvement in motor symptoms 30 minutes after taking Kynmobi, and an average of 7.6 points lower than placebo in the third part of the International Society for Motor Disorders' Unified Parkinson's Disease Assessment Scale (MDS-UPDRS).
15 minutes after the drug was given, clinical symptoms began to improve.
, patients treated with Kynmobi showed significantly higher rates of complete remission within 30 minutes than those receiving a placebo.
study, Kynmobi was well-to-do, with the most commonly reported adverse events caused by treatment (occurring in more than 5% of patients with a higher rate than placebo) including nausea, pharynx reactions, drowsiness and dizziness.
, Kynmobi is currently being developed as a quick-acting drug for on-demand treatment of all types of "OFF" events, including early morning OFF events, unpredictable OFF events, and end-of-dose effect OFF events.
the way Kynmobi is given under his tongue not only solves the problems caused by the injection under the skin, but also stabilizes the "OFF" symptoms of Parkinson's disease more quickly, and the safety is greatly improved.
PD patients (photo source: dovehomecare.com) Parkinson's disease treatment, several drugs have been approved in recent years, including: (1) Acorda's drug-device Inbrija, for the intermittent treatment of "OFF" events in PD patients treated with carbidoba/L-Doba, the first inhaled L-Doba product to be hand-operated by the patient.
(2) Concorde Kirin's Nourianz (istradefylline tablet), an additional therapy for zocondoba/carbidoba for PD adult patients undergoing OFF events, has been around for 11 years from its initial rejection by the FDA to its eventual approval.
(3) Akihon/Meiji Xadago/Equfina (safinamide), a new selective MAO-B inhibitor used in patients with Parkinson's disease who are receiving treatment with a ly-doba drug, improves efficacy reduction (wearing-off phenomenon).
(4) Neurocrine Biosciences, Inc. Ongentys (opicapone), a new, once-a-day, oral selective catechinol-O-methyl transferase (COMT) inhibitor, is used as an adjective therapy for patients with Parkinson's disease who are experiencing movement fluctuations (OFF events).
it is worth noting that Ongentys is the first and only approved daily COMT inhibitor that will provide a new treatment to extend the efficacy of L-Doba/Carbidoba.
January 2018, Fosun Pharma acquired exclusive rights to the Chinese market from BIOL for $18 million.
according to domestic research, the overall prevalence of Parkinson's disease in China over 65 years of age is about 1700/100,000, with nearly 100,000 new cases per year.
() Origin: Sunovion Announces The Commercial Launch of KYNMOBI (apomorphine hydrochloride) Sublingual Film for The Treatment of Parkinson's Disease OFF Episodes.