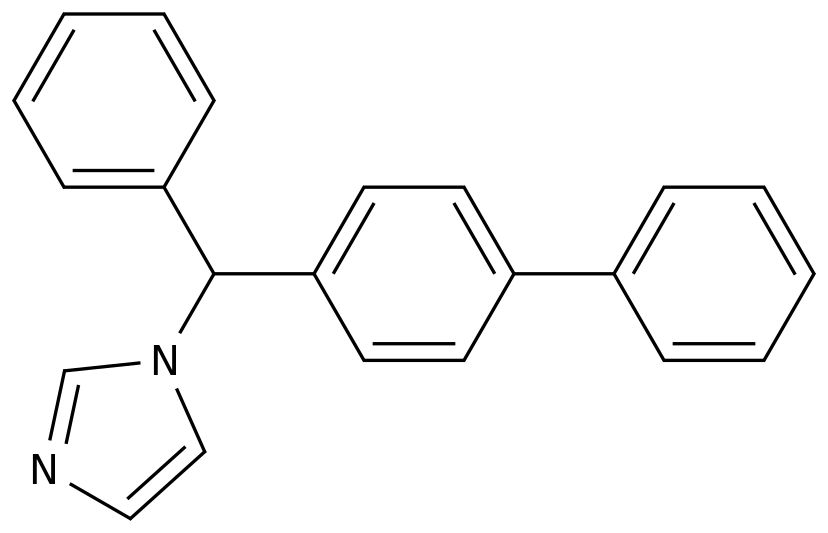
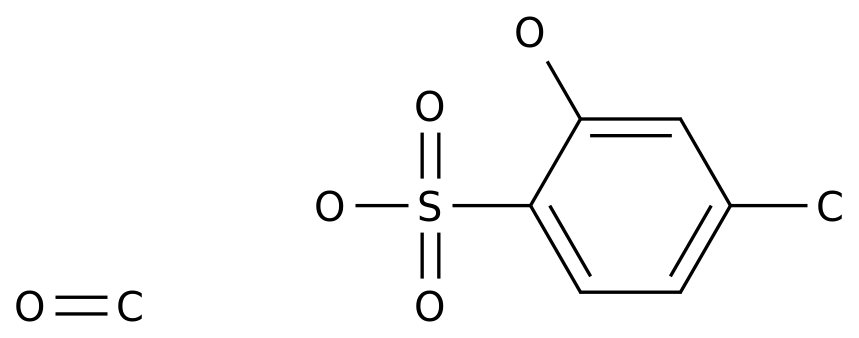
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
Pfizer Canada and BioNTech announced today that they are beginning to submit a rolling application to Health Canada for BNT162b2, a COVID-19 vaccine candidate.
BNT162b2 is based on BioNTech's proprietary mRNA technology and is supported by Pfizer's Global Vaccine Development Program.
BNT162b2, which encodes optimized SARS-CoV-2 full-length prickly proteins, is currently conducting Phase III clinical studies in more than 120 clinical sites worldwide, and to date, the trial has recruited approximately 37,000 participants, more than 28,000 of whom have received a second vaccination.
more information, please visit (NCT04368728).
submission has been accepted by an interim order from Canada's health minister, allowing Health Canada to begin its review as soon as information continues to pour in to speed up the overall review process.
the vaccine is approved, Health Canada will release evidence of its review to ensure transparency in the review process.
.