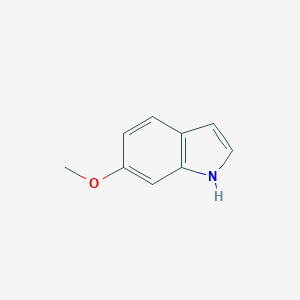
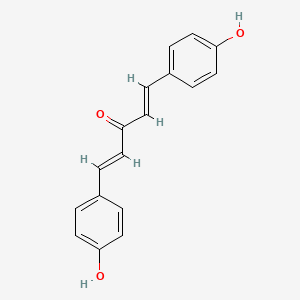
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
According to the following categories of new long-term anti schizophrenia preparations, Risperdal (Risperdal) developed and launched by Johnson & Johnson in 2003 In 2009, invega was a four week dose of paparone palmitate In the same year, olanzapine was given once every four weeks At present, the best-selling drug abilify is a four week dose of aripiprazole Risperdal is the first long-acting atypical antipsychotic agent, mainly used for continuous treatment and prevention of schizophrenia In 2011, the global sales of the drug reached US $1.6 billion The advantages of the product are good control of symptoms, higher vigilance, good tolerance, reduction of extravertebral motor symptoms, and less recurrence At present, green leaf has carried out the research and development of risperidone sustained-release microspheres for Risperdal in a new preparation without time delay, and has carried out clinical trials in China and the United States Since the clinical approval was obtained in January 2013, four single dose studies have been completed, and the clinical study is planned to be completed in mid-2015
The design concept of this project is as follows: first, the long-term sustained-release microsphere without drug release delay period, which does not need oral supplement after injection, takes effect quickly and achieves stable effect quickly, which will better improve the treatment effect of patients and overcome the defects of the original research drug; in addition, our product can be released slowly and continuously, intramuscular injection once every two weeks, which can maintain stable state The level of blood drug is consistent with that of the original drug, and the effect of inhibiting schizophrenia is continuously exerted