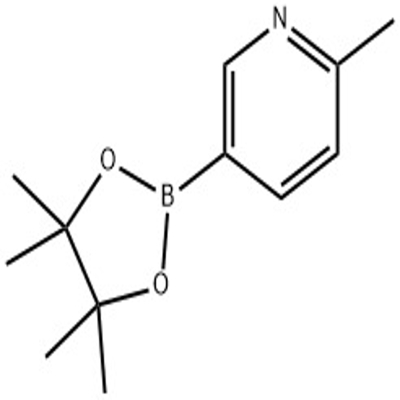
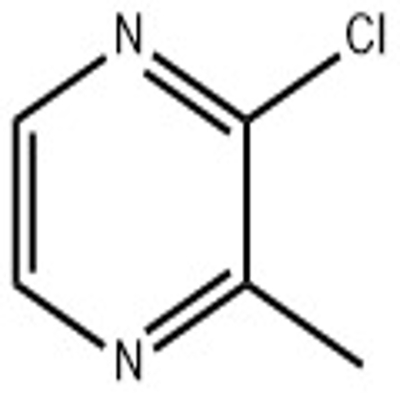
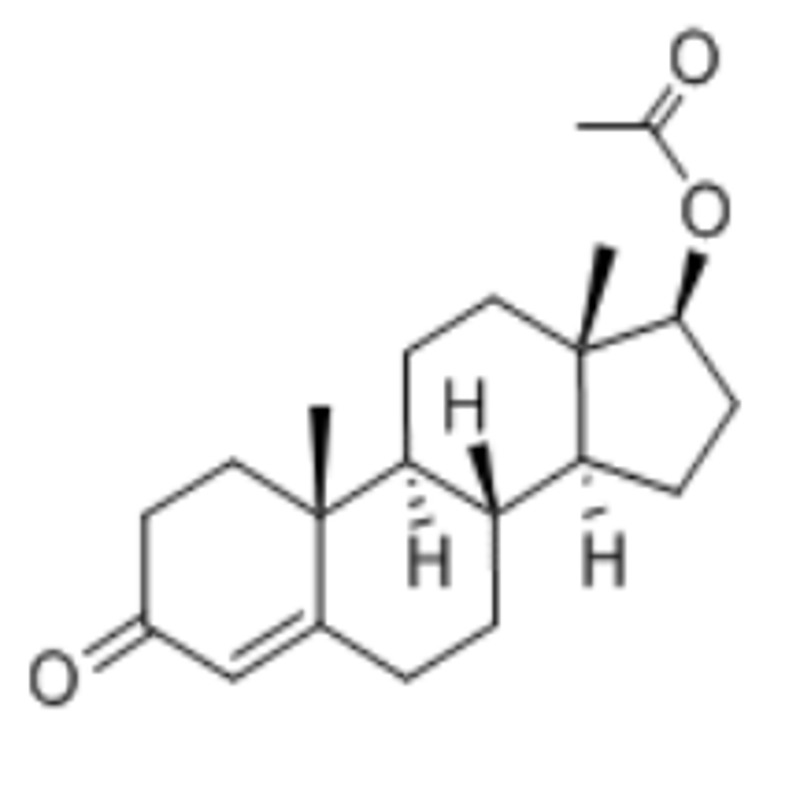
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
On the evening of August 14th, the Guangdong Provincial Health and Health Commission informed that Lufeng City, Yanwei, had detected a positive neocleic acid in the medical institution's fever clinic, and five new cases of local asymptomatic infection, all of which were closely followed by the above-mentioned cases.
August 15, Lufeng City, the new coronavirus pneumonia epidemic prevention and control command office issued a "on the suspension of retail pharmacy sales of "anti-fever medicine" notice", explicitly requires that from August 15, 2020 at 15:00, all retail pharmacies in the city suspended the sale of "anti-fever medicine" without a doctor's prescription.
notice requires retail pharmacies to strictly follow the spirit of the notice, suspend the sale of "anti-fever medicine" without a doctor's prescription, and guide customers who purchase "anti-fever medicine" to visit medical institutions.
to lift the suspension of sales without notice.
to the outbreak in Lufeng, Guangdong Province, on August 16, Zhong Nanshan academician said in an interview that Guangdong has responded quickly to the outbreak and has now carried out large-scale screening in the relevant areas of the outbreak.
his overall judgment, Lufeng this outbreak will not spread on a large scale, we do not need to panic.
announced that strict control of anti-fever drugs about pharmacies to stop the sale of anti-fever drugs, I believe you are no longer unfamiliar.
addition to the above-mentioned Lufeng City, there have been a number of recent notices to strengthen the supervision of pharmacies to sell anti-fever drugs.
Jingzhou, Hubei Province: Prohibition of the sale of fever to fever, fever medicine according to the official microblog of the press office of the Jingzhou Municipal People's Government of Hubei Province August 14 news, in order to strengthen the normal epidemic prevention and control work, effectively cut off the transmission of the virus, is now on the drug retail business related matters notice as follows: First, prohibit pharmacies to fever personnel to sell fever, cough and other drugs, to guide fever personnel to fever clinic.
to do a good job of heating personnel information registration, and timely report to the district prevention and control command and market supervision personnel.
, for those who do not have fever symptoms to sell cold, cough medicine, should register personal information, such as home address, contact telephone and other content, daily report to the district command and market supervision personnel.
, all fever personnel should take the initiative to the medical institutions fever clinic, free nucleic acid, antibody testing, so as not to miss the disease.
Heilongjiang: Strengthen the supervision of drugstores "anti-fever cough antiviral" drug August 12, Heilongjiang Drug Administration issued "on the continued strengthening of pharmacy sales of "anti-fever cough antiviral" drugs real-name registration supervision work notice", clearly requires to do a good job in the autumn and winter festival of the province's pharmacies to sell "anti-fever cough antiviral" drugs (hereinafter referred to as "three types of drugs") supervision work.
"Notice" requires to further improve the pharmacy sales of "three types of drugs" real-name registration of the importance of awareness, to promote pharmacies to improve the epidemic prevention and control "sentinel" sensitivity, so that prevention and control loopholes re-screening, prevention and control focus re-reinforcement, prevention and control work re-implemented.
Zhaoqing, Guangdong Province: secret visit spot checks, illegal cancellation of medical insurance fixed-point recently, Zhaoqing City, Guangdong Province, the outbreak prevention and control work command secret visit group to conduct random spot checks covert visits.
According to the covert visit group on the ground, the Municipal Market Supervision Bureau immediately interviewed the relevant drug retail enterprise heads, issued a notice of order rectification, requiring enterprises to implement the main responsibility, conscientiously implement the real-name drug purchase registration reporting system, the failure to implement the registration reporting system will be dealt with seriously in accordance with the law, and recommended that the Health Insurance Bureau cancel its medical insurance fixed-point pharmacy qualification.
Anhui Maanshan: anti-fever, cough, anti-diarrhea drugs, to sell with "code" recently, Anhui Province Maanshan City Market Supervision Bureau issued a notice, requiring retail pharmacies in the city to sell anti-fever, cough, anti-diarrhea drugs must continue to be sold through the city's digital epidemic control platform, not receiving two-dimensional code retail pharmacies may not sell the above drugs;
market regulatory authorities will check pharmacies from time to time, such as the inspection found that the drugstore has received the QR code does not pass the code, did not receive the QR code unauthorized sales of the above-mentioned drugs, will be strictly investigated.
.