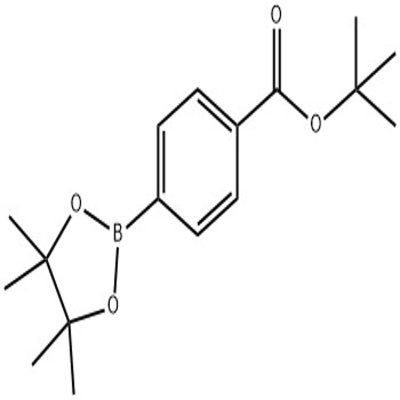
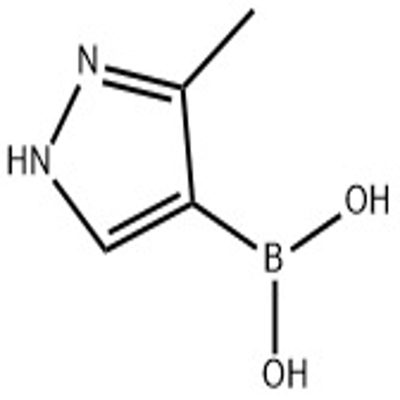
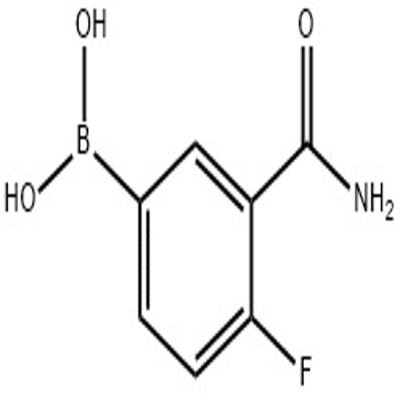
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
On march 3, the press office of the Shandong Provincial Government held a press conference and invited the relevant officials of the Shandong Provincial Food and Drug Administration to interpret shandong Province's Implementation Opinions on Deepening the Reform of the Review and Approval System to Encourage Innovation in Pharmaceutical Medical Devices (hereinafter referred to as the Opinions).
, Shandong Province, is a major province of the pharmaceutical industry, with the main business income of pharmaceutical industry enterprises above the scale of 459.897 billion yuan in 2017, up 12.81% YoY. But the problem of large but not strong industries is still prominent, according to data released by the Ministry of Industry and Information Technology, in 2016 there were 14 in Shandong, the top 100 pharmaceutical industry, and only one in the top 10. Product structure is not reasonable enough, biomedicine and other high value-added pharmaceutical drugs accounted for a low proportion, and low value-added raw materials high proportion. Insufficient innovation vitality, low level of foreign cooperation, the world's top 500 large multinational pharmaceutical enterprises, in Beijing, Shanghai, Jiangsu and other provinces and cities set up research and development centers or production bases, while Shandong Province is still blank. Ma Yueman, director of the Shandong Provincial Food and Drug Administration, said that the introduction of the Opinion is a rare opportunity to promote the transformation and upgrading of the pharmaceutical industry in Shandong Province, high-quality development, and accelerate the transformation from large to strong.
Opinions are divided into 5 parts and 20 specific measures to encourage innovation and development of the pharmaceutical industry, deepen the reform of the review and approval system, strengthen the management of the whole life cycle, enhance technical support capacity and improve safeguard measures. In optimizing the industrial structure, the Opinions propose to encourage pharmaceutical and medical device manufacturers to merge, restructure and develop jointly, and gradually cultivate a number of large enterprise groups with international competitiveness. Promote new products, new technologies and existing production capacity docking, and actively undertake advanced pharmaceutical manufacturing enterprises, innovative products, overall relocation projects, such as settled in Shandong. Implementation of international development strategy, the introduction of a number of international advanced technology, innovative products and global outstanding enterprises. Support the development of processing enterprises, to create a professional commissioned processing base. We will give full play to the guiding role of supporting funds in the pharmaceutical industry and transform and upgrade the traditional pharmaceutical industry. Implementation of the "quality of Lu medicine" construction project, cultivate a number of pharmaceutical brand products and well-known enterprises, to achieve high-end pharmaceutical industry brand.
In encouraging product innovation, the Opinion proposes to provide post-subsidy financial support for the first class of new drugs and improved new drugs that obtain new drug certificates and realize industrialization in Shandong Province, and to support key common technologies and major varieties in the development of pharmaceutical medical devices in provincial science and technology plans. We will improve the dynamic adjustment mechanism of the medical insurance drug catalogue, explore the mechanism for negotiating the standard payment of medical insurance drugs, and timely and timely incorporate new drugs into the scope of payment of basic medical insurance. Medical institutions are encouraged to give priority to the procurement and use of new drugs with clear efficacy and reasonable prices. Support leading backbone enterprises to actively create key laboratories, engineering and technology centers and other national platforms. We will accelerate the development of drugs for major diseases, as well as high-end and innovative medical devices. We will encourage marine innovation drug development, focus on breaking through key technologies for marine drug research and development, and build a complete marine drug innovation system.
In promoting the development of industrial aggregation, the Opinion proposes to speed up the creation of a number of new pharmaceutical industrial parks with standardized management norms, friendly environment, outstanding characteristics and high industrial relevance, and encourages enterprises to extend the industrial chain and promote the intensive development of the pharmaceutical industry on a large scale. Jinan, Zibo, Yantai, Luze and other pharmaceutical industry-intensive areas to play their own characteristics, highlight the development focus, the formation of distinctive characteristics, strong sustainable development capacity of the pharmaceutical industry group. We will accelerate the construction of key parks such as Jinan Pharmaceutical Valley, Yantai Life Sciences Innovation Demonstration Zone, Zibo Pharmaceutical Innovation Center and Weihai Medical Device Industrial Park, and strive to build a highland for the development of the province's pharmaceutical industry.
in promoting the transformation and upgrading of the circulation industry, the Opinion proposes to encourage mergers and acquisitions of enterprises with modern logistics conditions for pharmaceuticals, and to accelerate the formation of a drug circulation network supplemented by large-scale backbone enterprises and small and medium-sized enterprises. We will support the construction of national and regional drug logistics parks and distribution centers, and encourage enterprises with modern drug logistics conditions to carry out third-party drug logistics business. Promote the "Internet plus drug circulation". We will encourage retail pharmacy chain operations, promote the interconnection and real-time sharing of prescription information, medical insurance settlement and drug retail consumption information in medical institutions, and facilitate the purchase of prescription drugs for patients who need long-term use of fixed drugs.
in the optimization of services, "opinion" in the realization of the whole network, "up to run once" on the basis of the implementation of electronic license, and strive to "zero running legs." Further improve the approval of the "green channel", the implementation of early intervention, the whole process of help, accelerate the market for innovative pharmaceutical medical devices. Set up provincial clinical trial ethics committee, accelerate the construction of generic consistent evaluation service platform, build regional inspection sub-centers in industrial cluster areas, and extend the tentacles of technical services. Clearly apply only the traditional process of preparation of chinese medicine preparations in medical institutions to implement the filing system, improve the Shandong Province, Chinese medicine tablets preparation norms and other institutional norms. We will promote the application of medical data and encourage the development of intelligent Chinese medicine health service products.
, the Shandong provincial government will set up a joint meeting system with the vice-governor as the general convenor to comprehensively promote drug review reform, said Ma Yueman, a senior government minister. (Volkswagen.com)