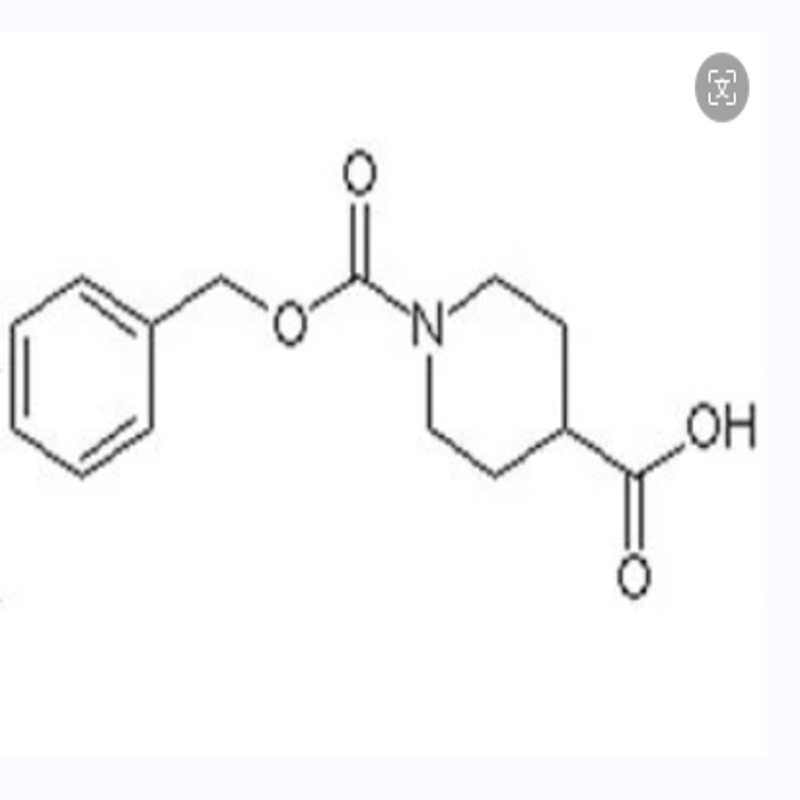
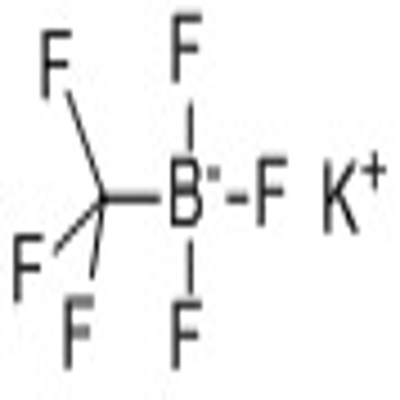
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
Recently, Shijiazhuang High-tech Industrial Development Zone issued a biopharmaceutical industry policy. It is reported that this policy applies to industrial and commercial registration and tax registration in Shijiazhuang High-tech District, and engaged in the field of biomedicine (including medical devices) research and development, production, circulation and services, with independent legal personality and with independent intellectual property rights or core technology of biopharmaceutical enterprises.article
If an enterprise obtains a batch of new drugs of the national large variety (according to the latest national drug registration classification standard) and settles the production on landing in our region, the enterprise shall be given subsidy support of up to 120 million yuan according to the production and sales situation.
2, enterprises are encouraged to declare special projects for the creation of major new drugs in the country, and to provide matching financial support with a ratio of not less than 1:1 to the central financial funds.Article
Article 3 For holders or teams who master the world's leading technology and have independent intellectual property rights to enter the high-tech zone and carry out the industrialization of scientific and technological achievements, the total investment amount shall be supported by a subsidy of 20%-30% in the course of landing to operation, up to a maximum of 50 million yuan.
Article 4 Provides phased funding for the progress of research and development of new drugs, 1 million yuan each for varieties that have completed preclinical studies and obtained acceptance numbers;
Article 5 For varieties studied by biopharmaceutical enterprises through in vitro consistency evaluation (different specifications are considered to be a variety), each variety supports a capital of 1 million yuan, and for varieties that complete in vitro consistency evaluation research and pass the in vivo bioethicity test (BE test), each variety supports 2 million yuan, and the number of varieties supported by each enterprise shall not exceed 3 in principle.Article
Article 6 For medical devices with independent intellectual property rights (including invention patents, software copyrights, etc.) who have obtained clinical approvals for three types of medical devices and entered clinical trials, each shall be supported by 2 million yuan;
500,000 yuan for research and development enterprises to obtain a Second Class Medical Device Certificate and settle production in our district.
Article 7 We will vigorously promote the construction of a public service platform for biopharmaceuticals, encourage biopharmaceutical enterprises to jointly build and operate a public service platform for biopharmaceuticals with professional companies through financial leasing and equity investment, and provide professional services such as analytical testing, product registration, CRO and GCP certification consulting to pharmaceutical enterprises in the region. The finance shall give 50% purchase subsidy to the newly acquired equipment of the biopharmaceutical public service platform each year, and shall not exceed 5 million yuan at a time.Article
Article 8 Where, in accordance with the system of the state drug listing license holder, the applicant for the drug market license holder is declared and obtained, and the industrialization is realized in our region, each variety shall be granted a capital subsidy of 10% according to the actual input of the holder, and the number of varieties applied for by each enterprise shall not exceed 10, and the total amount shall not exceed 5 million yuan.Article
Article 9 Encourages future industries and industry innovations such as precision medicine, cell therapy, immunotherapy, translational medicine, and personalized diagnosis and treatment, and for high-quality enterprises to rent incubator carriers to develop industries, the maximum proportion may be given for 3 consecutive years not more than 100%, the maximum annual maximum amount is not more than 2 million yuan rent subsidy, or for 6 consecutive years the annual subsidy is 50%, the annual maximum amount is not more than 1 million yuan rent subsidy.
Article 10 The biopharmaceuticals, medical devices or bio-monitoring projects in which enterprises focus on investment shall be rewarded according to the comprehensive situation of the assets of the enterprises, annual sales, project investment, number of employees, technical level and local taxes paid. If the actual fixed assets (excluding the project land) of an enterprise's new investment project reach more than 50 million yuan (or 7.5 million U.S. dollars of foreign capital), the amount of investment in the actual fixed assets (excluding the project land) shall be rewarded at 5% after completion acceptance and put into production, up to a maximum of 100 million yuan. If the investment projects in the new biopharmaceutical industry conform to the national industrial policies and quota targets for land supply, priority shall be given to the annual key project plan, which shall be guaranteed in the preparation of the annual plan for land use.
policy is explained by Shijiazhuang High-tech District Science and Technology Bureau and is valid for 3 years from the date of publication. Provisions of the previous policy that are inconsistent with this policy shall prevail.
on the development of biopharmaceutical industry in the high-techFirst, the status quo of the biopharmaceutical industry
(1) the basic situation of the biopharmaceutical industry
Shijiazhuang Hi-Tech Development Zone, as the first national bio-industry base, has now become one of the country's largest modern integrated bio-pharmaceutical industry base. In 2016, he was elected as the first chairman of the National Biopharmaceutical Industry Cluster (Park) Collaborative Innovation Alliance.
Pharmaceutical industry is the key development industry of Shijiazhuang High-tech Zone, accounting for more than 40% of the total output value of Shijiazhuang City pharmaceutical industry, accounting for more than 50% of Hebei Province, gathered more than 180 pharmaceutical enterprises, including Chinese medicine, stone medicine, Yiling, four drugs, Zhitong, Yanno and other well-known pharmaceutical enterprises, the overall technical level is strong, the industry's total labor productivity in the industry-leading level, products sold around the world.
High-tech zone pharmaceutical industry to highlight the development of biotechnology drugs, innovative development of chemical drugs, focusing on the development of new preparations and new accessories, enhance the development of modern Chinese medicine and Tibetan medicine, accelerate the development of medical equipment, actively cultivate bio-agriculture and health services, large-scale pharmaceutical circulation industry, extend the bio-pharmaceutical industry chain, build a high-end, diversified industrial structure system, become the most influential and competitive biopharmaceutical industry cluster.
(ii) The biopharmaceutical industry has significant advantages
1. Professional services, policy support
Shijiazhuang High-tech Zone attaches great importance to the development of innovation and entrepreneurship in the biopharmaceutical industry, has introduced a series of enterprise innovation and support policies, in the introduction of talent, fiscal taxation, enterprise land and other aspects of innovation and entrepreneurial enterprises have been given strong support. Has a number of well-functioning biomedical professional incubators, innovative drug research and development enterprise accelerator and biomedical research and development services outsourcing base.
2. Talent pool, seek common development
Shijiazhuang High-rise District has always adhered to the "talent development zone", "talent strong area" development strategy, talent resources as the first resource, has introduced a series of preferential policies to attract high-end talent. At present, the high-tech district has 16 academician workstations, 9 state-level postdoctoral workstations, the country's only innovative Chinese think tank academician research base. In 2016, Shijiazhuang International Talent City was officially opened. The whole region has more than 50 academicians, the national "thousand-person plan" experts 3, their own doctors and other types of high-end talent more than a thousand people.
innovation, the international advanced
Shijiazhuang High-tech Zone continues to strengthen the biopharmaceutical industry innovation platform construction, independent innovation capacity continues to improve effectively. We will vigorously introduce and build innovative platforms such as national key laboratories, enterprise research and development centers, engineering technology (research) centers and postdoctoral workstations, which are closely related to the development of leading industries in the region. Our district currently has enterprise technology center, engineering technology research center, engineering research center, key laboratory 108, including 4 national engineering research center, enterprise technology center 4, key laboratory 5. We attach great importance to the construction of new industrial technology innovation organizations. Support enterprises to join universities, scientific research institutions, the establishment of industrial technology research institutes, international innovation centers, industrial technology innovation alliances, technology transfer centers and other innovative organizations.
4. Leading, industry gathering
Shijiazhuang high-tech zone relying on the backbone of pharmaceutical enterprises, leading to the overall coordinated development of the entire biopharmaceutical industry chain. The park has 4 domestic pharmaceutical top 100 enterprises, listed 7 enterprises, pharmaceutical high-tech enterprises 45. The park has formed five major industrial sectors led by leading enterprises, namely, North China Pharmaceutical Microbiology and Bioengineering Pharmaceutical Industry, Stone Pharmaceutical Group's new preparation and accessories industry, Yiling Pharmaceuticals' modern Chinese medicine industry, Zhitong Group's biochemical drug industry, Yanno Bio's Tibetan medicine industry, forming a complete industrial chain from new drug research and development - incubation - industrialization - sales and services.
5. Innovation-driven, excellent results
Shijiazhuang High-tech District biopharmaceutical industry independent innovation results. Up to now, the park enterprises have undertaken more than 150 major national science and technology projects, and more than 400 new drug research projects, including a national class of new drugs and exclusive varieties of 33. Accumulated access to domestic invention patent licensing more than 2000, of which 56 applied for PCT patents. Built "North China", "Stone Medicine" two international well-known brands, with "North China", "Stone Medicine", "Yiling", "European", "Enbipu", "Go Wellcome", "Shimen", "Yanno" eight well-known trademarks in China, 31 well-known trademarks in Hebei Province. At present, the region has a total of 9380 patents, of which 2908 invention patents. 2, 2017 work highlights
(1) issued a series of policies
in order to further optimize the biopharmaceutical industry innovation and development environment in Shijiazhuang High-tech Zone, promote the scale, amassing and international development of the biopharmaceutical industry, accelerate the cultivation of the biopharmaceutical industry as an influential and innovative industrial cluster at home and abroad, the introduction of the Shijiazhuang High-tech Zone to promote the development of biopharmaceutical industry policy, Shijiazhuang High-tech Zone to promote the transformation of scientific and technological achievements, such as the implementation of the previous heavy-weight policy.
(ii) Set up a professional fund
the new high-tech district has recently set up a total size of 1 billion yuan of new drug fund and 5 billion yuan of industrial mother fund. The fund will focus on the health industry as the key investment direction, attract technology, talent and other high-end elements gathered, attract major health industry projects to land, promote the "medical, pharmaceutical, health" one-stop service platform construction, build an open and win-win health industry ecological environment, the construction of the country's leading health industry base.
(iii) The establishment of the Synergy Innovation Alliance
the establishment of the Shijiazhuang Medical and Pharmaceutical Industry Synergy Innovation Alliance is the high-tech zone integration and cultivation of medical institutions and pharmaceutical enterprises collaborative innovation and development of innovative major initiatives, the first time in the country. To guide and support the gathering of innovative elements to enterprises and medical institutions, accelerate the transformation of scientific and technological achievements, carry out innovative technology radiation, enhance the scientific and technological innovation capabilities of the biopharmaceutical industry, core competitiveness and comprehensive economic strength, and promote the healthy and rapid development of the biopharmaceutical industry.
(4) To build a sound public technical service platform and support system
Shijiazhuang High-tech District biopharmaceutical public technical service platform will be in accordance with the "government-led, based on the Internet and technical service platform, jointly with the city's backbone pharmaceutical enterprises, biomedical services outsourcing enterprises Clinical research hospitals in one of the "collaborative development model construction, aimed at shortening the research cycle of drugs, devices, integration of the biopharmaceutical industry chain on the advantages of scientific and technological resources, for biopharmaceutical research and development, enterprise technological innovation, biopharmaceutical industry development to provide professional, convenient, centralized technical support and services.
(v) To create an international biopharmaceutical industry park
In order to create an industrial aggregation effect, the high-tech zone plans to build an area of 2000 mu, construction area of 1 million square meters of "Shijiazhuang International Biopharmaceutical Industry Park", and strive to make the park the country's most influential and competitive 100 billion-class biopharmaceutical industry park, a service industry, industrial talent gathering international standard service platform, bearing the international responsibility for the rise of the biopharmaceutical industry. At present, the planning and design has been completed, in September this year officially started, looking forward to the global biomedical research and development, production and service institutions to claim customization.
(6) reserves a number of production capacity cooperation carriers
At present, the region has a total of various types of shared plant, workshop and storage facilities 160,000 square meters, including 30,000 square meters of plant, GMP workshop 110,000 square meters, 20,000 square meters of pharmaceutical storage, warmly welcome all kinds of biomedical research and development and production institutions to discuss cooperation and seek common development. (Phoenix Network Hebei)