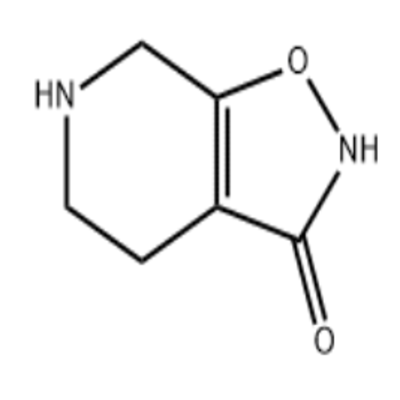
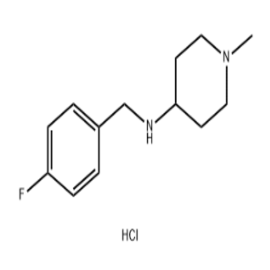
State Food and Drug Administration issued document No.1 real world evidence to support drug regulation
-
Last Update: 2020-01-08
-
Source: Internet
-
Author: User
Search more information of high quality chemicals, good prices and reliable suppliers, visit
www.echemi.com
Guide reading On January 7, the State Food and Drug Administration issued the guiding principles of real world evidence supporting drug development and review (Trial) This is considered a milestone event, and real world research (RWE) will be an important direction of drug research and development For new drugs, RWE can reduce the cost of phase 3 clinical trials to a certain extent; for drugs with expanded indications, phase 3 clinical trials can be directly exempted Drug access for rare diseases, approval of new indications for broad-spectrum drugs (such as PD-1 inhibitors), and reevaluation of traditional Chinese medicine are believed to benefit the most However, clinical trials will still be the mainstream of new drugs on the market, and the real world evidence is complementary and complementary The real world data base is not enough, and the data guidance rules are under discussion △ image source: yestone On the evening of January 7, the National Drug Administration (nmpa) issued the first document in 2020, "guiding principles of real world evidence supporting drug research and development and review (Trial)" Both the industry and the academic circles have a high evaluation of it "This is a landmark event, because it is a great change from the regulatory concept," said Xuan Jianwei, director of the Institute of pharmaceutical economics, School of pharmacy, Sun Yat sen University Jin Chunlin, director of Shanghai Health and health development research center, also pointed out that "real world research / study (RWR / RWS) has adapted to the current direction of scientific development." "Real world research will be an important direction of drug research and development," said Luo Ligang, co-founder and chief operating officer of zero krypton technology As early as last May, when the draft was released, it had caused great shock in the pharmaceutical industry In terms of reducing the cost of drug research and development, "real world research can be said to be one of the few innovative directions." Pharmaceutical companies welcome real world evidence Worldevidence, RWE), a manager of a multinational pharmaceutical company, believes that "there have been many drugs approved with new indications through real world data before", "in the past, we will consider real world data when there may be no way to do it With the policy, the big family will be more convenient or discuss the use of real world evidence earlier." Real world research is an evidence-based medicine research method corresponding to randomized controlled trial (RCT) In short, RWS mines information from real world data (RWD) other than traditional evidence-based clinical research, adopts non random, open and placebo free research, and obtains real world evidence through analysis to support regulatory decision-making of medical products The 21st century cure act, passed in December 2016 in the United States, is generally regarded as the starting point for real-world evidence to support regulatory decisions on drugs and other medical products and accelerate the development of medical products After this, RWS has become a global regulatory body, pharmaceutical industry and academia of common concern and very challenging issues The release of the guiding principles by the State Food and drug administration is only three years later than that of the United States China's innovation in new drug review and approval is faster and faster Drug access for rare diseases, approval of new indications for broad-spectrum drugs (such as PD-1 inhibitors), and reevaluation of traditional Chinese medicine are considered to be the fastest way to benefit from the guiding principles △ real world research paths to support drug regulatory decisions (solid line) Source: guiding principles for real world evidence supporting drug development and review (Trial) The guiding principles provide five application areas for real world evidence to support drug regulatory decisions, the fifth of which is the others, while the first four are very specific: (1) To provide evidence of effectiveness and safety for the registration and listing of new drugs; (2) Provide evidence for the change of the specification of the marketed drugs; (3) Provide evidence for post marketing requirements or re evaluation; (4) The experience summary and clinical research and development of traditional Chinese medicine prescription and preparation of traditional Chinese medicine medical institutions For the first, people with rare diseases are considered to be the most likely beneficiaries About 7000 kinds of rare diseases are known in the world, of which only about 6% have drugs to treat The world is speeding up the research and approval of rare disease drugs There are about 20 million rare patients in China In May 2018, the state released the catalogue of the first batch of rare diseases, including 121 rare diseases, affecting about 3 million patients As of December 2018, there are 162 therapeutic drugs in 74 of them in the United States, the European Union, Japan and other places Only 83 of them are listed in China, and 53 rare diseases can be treated In 2019, nmpa approved 8 new drugs for rare diseases to treat 8 kinds of rare diseases, 6 of which are included in the first batch of rare diseases catalogue Every rare disease drug almost represents the survival hope of a group A few days ago, in the message of the article "the state health insurance bureau answers the proposal of rare diseases intensively, and continues to include orphan drugs in the medical insurance next", there are many types of patients with rare diseases calling for new drugs However, half of the rare disease drugs approved by foreign countries have not entered China The important reason is that clinical trials are difficult to complete Traditional clinical trials need an experimental group and a control group Rare patients are rare, so it is the biggest challenge to recruit enough patients But if we can use the real world data to show the natural characteristics of the disease, and compare it with the experimental group, we can recruit half of the patients less, which will undoubtedly greatly reduce the cost of drug companies, and accelerate the registration of new drugs for rare diseases in China In addition, for drugs with a wide range of indications, such as PD-1 inhibitors, there are usually many super indications after the new drugs are launched How to rapidly expand more indications, real world research is a very good method △ image source: yestone There is a precedent for drugs to be approved for new indications through real world data The case of bevacizumab was published in the guiding principles Bevacizumab is a humanized monoclonal antibody preparation of vascular endothelial growth factor (VEGF) In 2015, bevacizumab was approved in China as a combination chemotherapy (carboplatin and paclitaxel) for the first-line treatment of unresectable patients with advanced, metastatic or recurrent NSCLC In October 2018, the State Food and drug administration expanded the indications of bevacizumab from "chemotherapy combined with carboplatin and paclitaxel" to "combination of platinum based chemotherapy (including cisplatin, carboplatin + pemetrexed, gemcitabine, albumin paclitaxel, paclitaxel, vinorelbine and other common drugs)." The evidence behind this is the real world data from three hospitals: Shandong Cancer Hospital, Jiangsu cancer hospital and Cancer Hospital of Chinese Academy of Medical Sciences Taking Shandong cancer hospital as an example, in a real world retrospective study of 1352 patients, the median total survival time of patients receiving bevacizumab + chemotherapy was longer than that of patients receiving chemotherapy alone for 3 months, and adverse reactions were tolerable △ source: real world research guide 2018 (written by Wu Yilong, chairman of cancer medicine department of Wu Jieping medical foundation, organized by relevant experts) The most direct value of real-world evidence for drug research and development is to reduce costs New drug development has a long time, high cost and low success rate Many years ago, there was a saying that the average R & D of a new drug was 10 years and the cost was 1 billion US dollars In recent years, the cost is still rising He Ruyi, a former FDA approval officer and chief scientist of the National Drug Review Center (CDE), once pointed out that the current trend of high cost of drug research and development is unsustainable, and new ways must be used to reduce the cost of drug research and development One of the ways is to use real world data to support the review, which can partially replace the clinical trials in drug research and development and save the cost of drug research and development This is also one of the reasons why pharmaceutical companies welcome real world evidence The managers of the aforementioned multinational pharmaceutical companies said that RWE can reduce the cost of phase 3 clinical trials to a certain extent for new drug research and development; for drugs with expanded indications, phase 3 clinical trials can be directly exempted "For enterprises that meet the requirements of RWE, its cost and time will be dramatically reduced, especially for the development of broad-spectrum drug indications It is very good to use RWE", but Luo Ligang believes that RCT will also be the mainstream of new drug marketing, and RWE as an auxiliary and supplement "At the beginning of the policy, if pharmaceutical companies can afford RCT, they may still prefer RCT After all, RWE is a new thing, which needs to be further verified." Luo Ligang even asserted that "after the implementation of the policy, many enterprises will try to launch RWE research, but eventually can run down, can really get effective and reliable evidence, not too much." The reason for this assertion is that the real world data (RWD) as the premise of RWE is not enough Real world research, some of which are forward-looking and some of which are retrospective, is based on historical data for registration approval The research based on historical data does not entirely depend on the ability of the enterprise itself, more importantly, the availability of current data, and whether their quality can meet the needs of registration In the past, the data in the medical system was not for the registration needs, but mainly for the regulatory needs For example, the primary purpose of the medical insurance system is to meet the medical insurance as the task of payment supervision However, the real world research needs the data of registration application, evaluation effect, product effectiveness and safety The data richness of the original system is not enough When he saw the newly released guiding principles, he was surprised by the fact that he put forward a large number of statements about real world data, including the evaluation of data relevance and reliability, as well as the introduction of statistical methods and research methods "This shows that the regulatory authorities are very down-to-earth in policy-making." If the introduction of the guiding principles is to establish norms for the application of real world evidence, then more detailed rules and standards will be needed for the smooth implementation of the policy According to Luo Ligang, the guiding rules on real world data are also being discussed and pushed forward, "what kind of real world data can obtain real world evidence, and then expand the indications" Jin Chunlin also believes that with the implementation of the policy, there will be more detailed "rules of the game", and the formulation of the rules not only needs to be led by CDE, but also requires CDE to launch the power of expert network to develop together, including research hospitals, national research centers, etc should participate in the development together In September 2019, there is a short video hot in the network Xiaoya, an 8-year-old girl from Xuchang City, Henan Province, ran to the drugstore and said she wanted to buy Viagra She bought 10 boxes of Viagra, 500 yuan each It's a bit awkward It's been staged in recent years In the face of other people's different eyes, Xiaoya's mother can only explain again and again with tears - her daughter is a patient with pulmonary hypertension, and taking Viagra can save her life Xiaoya suffered from idiopathic pulmonary hypertension, which is one of the first rare diseases in the catalogue This kind of patients are very young, but their quality of life is very low Once diagnosed, their average life span is less than three years, their appearance is sound, but their movement is limited Their daily walking is also a kind of painful torture for them There are dozens of drugs for pulmonary hypertension
This article is an English version of an article which is originally in the Chinese language on echemi.com and is provided for information purposes only.
This website makes no representation or warranty of any kind, either expressed or implied, as to the accuracy, completeness ownership or reliability of
the article or any translations thereof. If you have any concerns or complaints relating to the article, please send an email, providing a detailed
description of the concern or complaint, to
service@echemi.com. A staff member will contact you within 5 working days. Once verified, infringing content
will be removed immediately.