The first innovation in 25 years! The use of oral CGRP receptor antagonist ubrogepant in migraine treatment will be approved by FDA next month!
-
Last Update: 2019-11-21
-
Source: Internet
-
Author: User
Search more information of high quality chemicals, good prices and reliable suppliers, visit
www.echemi.com
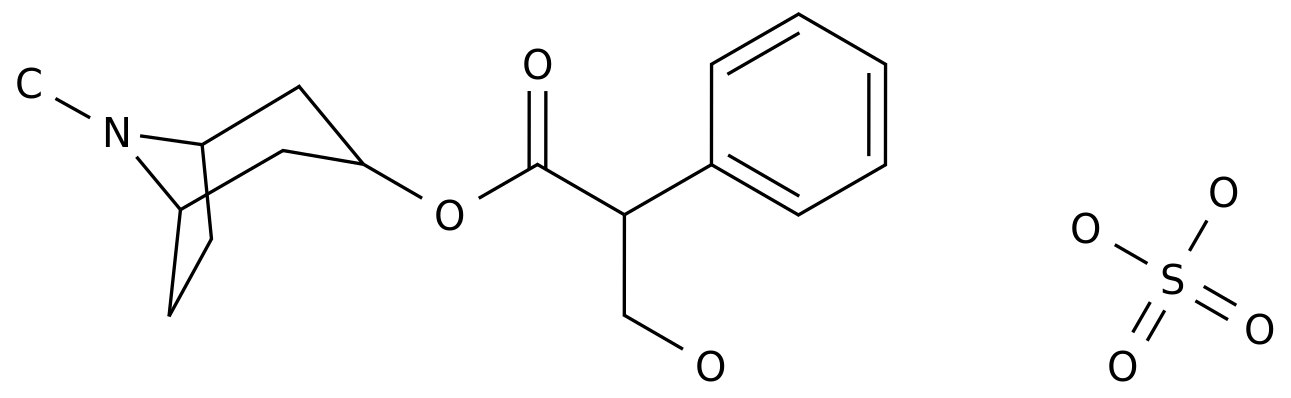
November 21, 2019 / BIOON / -- Allergan recently announced that the positive results of evaluating the application of ubrogepant in the phase III clinical study of adult migraine acute treatment acieve II (ubr-md-02) have been published in the Journal of the American Medical Association (JAMA) published on November 19 These data further confirmed that in terms of acute migraine treatment, patients treated with two doses (50mg and 25mg) of ubrogepant had significantly higher rates of pain free patients at 2 hours after administration and patients treated with 50mg of ubrogepant had significantly higher rates of migraine free most annoying symptoms at 2 hours after administration compared with placebo Currently, the new drug application (NDA) of ubrogepant for acute treatment of adult migraine is under review by the US FDA, which will make a review decision in December this year If approved, ubrogepant will be the first oral calcitonin gene-related peptide (CGRP) receptor antagonist on the U.S market for acute migraine (with or without aura) treatment in the past 25 years Achieve II is a multicenter, double-blind, parallel group III study that evaluated the efficacy, safety, and tolerability of ubrogepant (50mg and 25mg) for acute treatment of single migraine attack with moderate or severe headache pain intensity at home compared to placebo In this study, 1355 adult patients (18-75 years old) with migraine history (with or without aura) were randomly assigned (1:1:1) to receive placebo, ubrogepant 25mg and ubrogepant 50mg The common main efficacy parameters of the study were pain relief (no pain) 2 hours after the initial dose and no most annoying migraine related symptoms 2 hours after the initial dose The most annoying migraine related symptoms may include photophobia, voice phobia, or nausea, selected by the subject at the time of migraine onset The secondary efficacy endpoint also assessed the clinical benefits of ubrogrepant in a range of outcome measures, including 2-hour pain relief, 2-24-hour continuous pain relief, and 2-24-hour continuous pain relief (no pain) Adverse events were collected and evaluated 48 hours after the first and optional second administration and 30 days after any one administration The results showed that in terms of two common primary endpoints, the proportion of patients who achieved pain relief (no pain) 2 hours after the initial administration was significantly higher in the two doses (50mg and 25mg) of ubrogepant than in the placebo group, and the data were statistically significant (21.8% in the 50mg group, 20.7% in the 25mg group, 14.3% in the placebo group), and the 50 mg dose of ubrogepant after the initial administration The proportion of patients without the most annoying migraine related symptoms within 2 hours was higher and the data was statistically significant (38.9% in the 50mg group and 27.4% in the placebo group) In addition, in terms of the proportion of patients who achieved photophobia elimination (no photosensitivity: 43.8% in the 50mg group, 35.5% in the placebo group) and voice fear elimination (no voice sensitivity: 54.1% in the 50mg group, 46.3% in the placebo group), the 50mg dose of ubrogepant treatment group was statistically superior to the placebo group In this study, both 50mg and 25mg doses of ubrogepant were well tolerated, and the characteristics of adverse events were similar to those of placebo The most common adverse event in this single episode study (incidence unit 2%, and at least twice as high as placebo) was nausea (incidence 2-4%) The most common treatment-related adverse event in the one-year extended study was nausea, with an incidence of < 2% At present, triptan is the most commonly used drug in the treatment of migraine, accounting for 70% of the prescription However, about 20% of migraine patients have multiple cardiovascular (CV) risk factors, and tritan drugs have vasoconstrictive effects, which may not be suitable for migraine patients with CV risk factors As many as 30% of patients taking traptan may not respond well or may have side effects In the achieve II study, about 11% of the patients had moderate to severe CV risk factors, and 23% of the patients had insufficient response to traptan before "If approved, ubrogepant will provide a new, different acute treatment for patients with debilitating pain and other migraine symptoms," said Mitchell Mathis, MD, vice president of central nervous system at Elgin Elgin has a long history in the development of migraine drugs and is still committed to the continuous innovation of new therapies Ubrogepant is a new acute migraine therapy that will expand the company's migraine portfolio, including Botox, the first FDA approved preventive treatment for adult chronic migraine We will also continue to promote the phase III clinical project of atogepant, which is our second oral medication specifically designed to prevent migraine Since not every patient responds to every drug, we believe that patients need to have a variety of different treatment options " The molecular structure formula of ubrogepant (picture source: Wikipedia) in the United States, the new drug application (NDA) of ubrogepant is based on the successful completion of 4 clinical studies 2 key phase III studies (ACHIEVE I, ACHIEVE) 2) And two additional safety studies (ubr-md-043110-105-002) that demonstrated the efficacy, safety, and tolerance of ubrogepant for acute migraine treatment in a wide range of patient groups, including patients with inadequate response to traptan, patients with contraindications to traptan, and patients with moderate to severe cardiovascular risk characteristics Ubrogepant is a new, efficient, oral calcitonin gene-related peptide (CGRP) receptor antagonist, which has been developed for the treatment of acute migraine CGRP and its receptor are expressed in the nervous system related to the pathophysiology of migraine CGRP receptor antagonism is a new mechanism of acute migraine treatment, which is different from the existing triptans (serotonin 1B / 1D agonists) and opioids At present, CGRP receptor has become a hot target of migraine drug development So far, three McAbs targeting CGRP receptor have been listed, respectively: Novartis / ammovig (erenumab aooe), ajovy (fremanezumab vfrm) and emgality (galcanezumab gnlm) In terms of medication, aimovig and emgality are injected subcutaneously once a month, ajovy can be injected subcutaneously once a month or once every three months, which is more convenient in terms of medication and will provide patients with a differentiated treatment option In addition, the company's monoclonal antibody eptinezumab (intravenous infusion once in March) also filed an application for listing in the United States in late February this year Other companies are developing oral CGRP inhibitors, including biomegepant from biohaven, in addition to ubrogepant and atogepant from Elgin Original source: 1 Allegan announces positive phase 3 achievement II trial results for ubrogepant published in the Journal of the American Medical Association 2 Effect of ubrogepant vs placebo on pain and the most bosome associated symbol in the actual treatment of migration: the achievement II standardized clinical trial
This article is an English version of an article which is originally in the Chinese language on echemi.com and is provided for information purposes only.
This website makes no representation or warranty of any kind, either expressed or implied, as to the accuracy, completeness ownership or reliability of
the article or any translations thereof. If you have any concerns or complaints relating to the article, please send an email, providing a detailed
description of the concern or complaint, to
service@echemi.com. A staff member will contact you within 5 working days. Once verified, infringing content
will be removed immediately.