-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
-
Cosmetic Ingredient
- Water Treatment Chemical
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
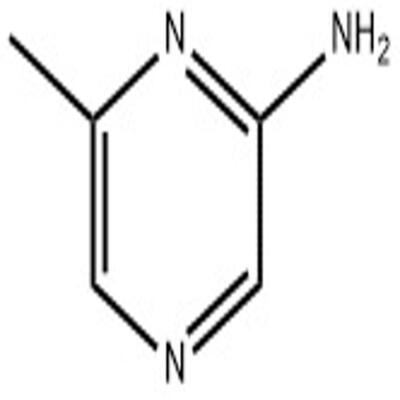
The Chemical Synthesis of 2-Hydroxy-4-(trifluoromethyl)pyrimidine: A Comprehensive Overview
As the chemical industry continues to grow and evolve, new compounds with unique properties are being discovered and synthesized at an increasing rate.
One such compound is 2-hydroxy-4-(trifluoromethyl)pyrimidine, a molecule with a distinctive structural formula and a range of potential applications.
In this article, we aim to provide a comprehensive overview of the synthesis of 2-hydroxy-4-(trifluoromethyl)pyrimidine, from the basic chemical reactions involved to the latest advances in industrial-scale production.
Whether you are a chemist, chemical engineer, or simply interested in the latest developments in the field, this article is sure to provide valuable insights into the complex world of chemical synthesis.
Introduction to 2-Hydroxy-4-(trifluoromethyl)pyrimidine
2-hydroxy-4-(trifluoromethyl)pyrimidine is a synthetic organic compound that is structurally classified as a pyrimidine.
Pyrimidines are a class of molecules that contain a six-membered aromatic ring with two nitrogen atoms, one of which is double-bonded to a carbon atom.
2-hydroxy-4-(trifluoromethyl)pyrimidine differs from other pyrimidines in that it contains a hydroxyl (-OH) group attached to the 2-position of the pyrimidine ring, as well as a trifluoromethyl (-CF3) group attached to the 4-position.
The trifluoromethyl group makes 2-hydroxy-4-(trifluoromethyl)pyrimidine highly resistant to chemical reactions that would otherwise be degradative, and this property makes it particularly attractive for use in various industrial applications.
Additionally, the presence of the hydroxyl group makes 2-hydroxy-4-(trifluoromethyl)pyrimidine a versatile building block for the synthesis of a wide range of compounds.
Synthesis of 2-Hydroxy-4-(trifluoromethyl)pyrimidine
The synthesis of 2-hydroxy-4-(trifluoromethyl)pyrimidine typically involves several chemical reactions, each of which requires careful control and monitoring to ensure the production of a high-purity product.
The following steps provide a general overview of the synthesis process:
- Preparation of the starting materials: The synthesis of 2-hydroxy-4-(trifluoromethyl)pyrimidine typically begins with the preparation of a number of starting materials, including the appropriate pyrimidine base, a suitable trifluoromethylation reagent, and any other reagents that may be necessary.
- Pyrimidine base synthesis: The pyrimidine base is typically synthesized through a series of reactions that involve the conversion of a starting material, such as anthranilic acid or 2,4-diaminotoluene, into the desired pyrimidine structure.
- Trifluoromethylation: The pyrimidine base is then treated with a trifluoromethylation reagent, such as trimethylsilyl trifluoride or hexamethyldisilazane, to introduce the trifluoromethyl group into the molecule.
- Hydroxylation: Finally, the trifluoromethylated pyrimidine is treated with a hydroxylation reagent, such as sod