-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
On January 10, the State Drug Administration issued an announcement officially approving the 1.
2-class innovative traditional Chinese medicine icariin (acoladine) soft of Beijing Shennuoji Pharmaceutical Technology Co.
, Ltd.
, a subsidiary of Beijing Shengnuoji Pharmaceutical Technology Co.
, Ltd.
Application for marketing registration of capsules
.
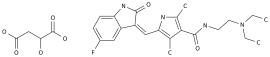
Icariin (Acoradine) Soft Capsule is a first-in-class Chinese medicine (first-in-class) developed by Beijing Shengnuoji Pharmaceutical Technology Co.
, Ltd.
for 15 years.
Professor Qin Shukui from the Qinhuai Medical District of the Eastern Theater General Hospital is the chief expert, through a randomized controlled, double-blind and double-simulation, national multi-center phase III clinical study of superiority
.
Clinical trial data show that: icariin (acoladine) soft capsules are suitable for patients with unresectable hepatocellular carcinoma who are not suitable or who refuse to receive standard treatment and who have not received systemic systemic therapy before.
Markers meet at least two of the following detection indicators: AFP≥400 ng/mL; TNF-α<2.
5 pg/mL; IFN-γ≥7.
0 pg/mL
.
PART.
01 patients have significant survival benefit, and the safety advantage is outstanding.
Icariin (Acoradine) soft capsule is the first small-molecule immunomodulator (content 98.
0-102.
0%) derived from the traditional Chinese medicine Epimedium
.
Studies have shown that acoradine inhibits the TLR-MyD88-IKK-NF-κB inflammatory pathway by directly binding to MyD88/IKKα, thereby reducing the production of inflammatory factors such as TNF-α and IL-6, and down-regulating the IL6-JAK2-STAT3 pathway
.
At the same time, it directly binds to IKKα to inhibit the activation of TNF-α on the IKK-NF-κB signaling pathway, thereby inhibiting the expression of PD-L1 and the effect of MDSC, activating IFN-γ positive CD8+ T cells, and exerting anti-tumor effect.
.
Its phase II clinical study showed that acridine can improve overall survival (OS) in patients with advanced hepatocellular carcinoma compared with historical control data, and overall survival was significantly correlated with immune biomarkers
.
Phase III clinical trials use composite biomarkers and adaptive enrichment designs
.
Among the 283 patients enrolled in the whole population, the enriched population (that is, patients whose peripheral blood composite markers meet at least two of the following detection indicators: AFP≥400 ng/mL; TNF-α<2.
5 pg/mL; IFN-γ≥7.
0 pg /mL)) 71 cases
.
Among them, there were 33 cases in the acoladine group and 38 cases in the cinobufacin group
.
After analyzing 20 risk factors associated with severe baseline and poor prognosis, such as BCLC stage C, tumor burden accounted for 50%, platelet count 75x109/L, AFP≥400ng/mL, etc.
In the enriched population, the proportions of subjects meeting all three risk factors were 94% and 97%, respectively
.
The median follow-up time of the study was 8.
1 months, and the median OS of the enriched population in the acoladine group and cinobufacini group was 13.
54 and 6.
87 months, respectively (hazard ratio HR=0.
43, 95%CI 0.
23-0.
82, p=0.
0092 ), investigator-assessed median TTP was 3.
65 vs 1.
84 months (hazard ratio HR=0.
67, 95%CI, 0.
36-1.
22, p=0.
1665), DCR was 48.
5% (30.
8%-66.
5%) and 26.
3 % (13.
4% - 43.
1%), p=0.
0712
.
Median time to deterioration (mTTD) based on quality of life (QOL) scale assessment was 7.
3 vs 2.
8 months in the enriched population of the two groups (HR=0.
43, 95%CI 0.
19-0.
98, p=0.
039) , 7.
3 and 3.
7 months in the full population, respectively (HR=0.
66, 95% CI 0.
44-0.
98, p=0.
037)
.
In terms of safety, the acoladine group had a lower incidence of various adverse events compared with the cinobufacini group: the incidence of adverse events related to the study drug in the whole population was 61.
7%: 78.
7%, grade ≥3.
The incidence of adverse events related to the study drug was 12.
1%: 26.
2%, the incidence of adverse events related to the study drug leading to permanent discontinuation was 0.
7%: 2.
1%, the incidence of adverse events related to the study drug leading to dose adjustment The incidence of serious adverse events related to the study drug was 4.
3%:17.
7% and 0:5.
7%
.
The safety results observed in the enriched population were similar to those in the full population
.
PART.
02 is close to the needs of Chinese patients and fills the precise treatment plan for advanced hepatocellular carcinoma.
In recent years, many breakthroughs have been made in clinical research on systemic treatment drugs for hepatocellular carcinoma, including targeted and immunotherapy.
However, in international and domestic clinical practice of advanced hepatocellular carcinoma, the prognosis There are currently no standard or optimal first-line treatment options for patients with poor advanced hepatocellular carcinoma
.
Most patients with HCC in China are diagnosed with advanced liver cancer, and more than 80% have a history of HBV infection.
Relative to some patients with advanced HCC, the constitution is poor and often accompanied by multiple underlying diseases and prognostic risk factors, such as severe liver function damage.
, the immune function is abnormal, the side effects of the existing first-line treatment are generally large, and the tolerance is poor
.
These patients have high bilirubin, low platelets, and poor liver function in clinical treatment.
When using existing chemotherapy and targeted drug therapy, they generally have poor prognosis and high toxic and side effects (grade 3 and 4 adverse effects).
response)
.
At present, in domestic clinical practice, some doctors use traditional Chinese medicine preparations as alternative medicines, but most of these medicines lack the support of high-quality, randomized, controlled, and multi-center clinical trials
.
"Icaritin (Acoradine) Soft Capsules, as a small molecule immunomodulatory drug, can significantly prolong the overall survival and improve the quality of life of the advanced hepatocellular carcinoma-enriched population with heavier baseline and poor prognosis, and Has significant clinical safety advantages
.
"Professor Qin Shukui pointed out that the icariin (acoladine) research plan fully draws on the latest international clinical enrichment design guidelines and the successful experience of published enrichment design (REACH2) clinical research, combined with the clinical characteristics of Chinese hepatitis B virus.
The precise enrichment design of the heterogeneous characteristic markers in patients with poor prognosis has promoted the precise clinical research and treatment exploration of advanced hepatocellular carcinoma; Advanced hepatocellular carcinoma patients provide new solutions that are closer to Chinese patients, and also open up new directions for the inheritance, innovation and exploration of local innovative drugs, especially traditional Chinese
medicine
.
Combining with biotechnology, a biopharmaceutical enterprise focusing on drug research and development in the field of tumor treatment, Shengnuoji has always been the focus of attention in the field of pharmaceutical investment.
Lin Capital, Tus Venture Capital, Shenzhen Venture Capital, Legend Capital, China Life and other venture capital companies have invested more than 1 billion yuan in total
.
With continuous high-intensity investment, the scientific research system of Shengnuoji has been increasingly improved
.
The company has passed the Introduced talents and team building, developed and constructed natural small molecule drug development platform, synthetic small molecule drug research and development platform, biological macromolecule new drug research and development platform and estrogen receptor target research and development platform; has a number of national-level specially-appointed experts, provincial (city) )-level specially-appointed experts, some core technicians have more than 20 years of experience in new drug research and development, and have the experience of leading or participating in the successful launch of multiple new drugs, providing support for high-quality innovative research and development
.
Dr.
Meng Kun, chairman of Shengnuoji Pharmaceutical, introduced , In recent years, the company's research and development projects have adhered to the orientation of clinical needs, focusing on multiple malignant tumor fields with urgent clinical needs and good market prospects, and committed to the development of First in Class or Best in Class.
innovative drugs
.
At present, the company has 20 research projects with 12 products under research, covering hepatocellular carcinoma, breast cancer, non-small cell lung cancer, lymphoma and other fields.
Among them, the two indications of the research drug S NG1005 have obtained II approvals respectively.
/ Approved in Phase III and Phase III clinical trials, and the drug under investigation, fluocoradine, has been approved in Phase I clinical trials, and has also been approved for clinical trials in the United States
.
The remaining 9 drug candidates are in the preclinical research stage
.
In terms of intellectual property protection, the company has obtained 57 invention patent authorizations, including 26 authorized in mainland China and 31 authorized overseas
.
"Shengnuoji has always been based on better solving the clinical needs of domestic patients as the starting point and foothold of research and development
.
" Dr.
Meng Kun introduced that icariin (Acoradine) was used as the core structure to transform and optimize.
A chemically synthesized derivative, fluocoradine, is a brand-new tumor immunomodulatory small molecule drug, intended for use in patients with advanced hepatocellular carcinoma.
As part of the company's promotion of product internationalization, the company is developing the first The second-generation formulation (SNG168), using nano-polymer mixed micelles (PMM) as a new drug delivery system, can deliver drugs to target tissues more precisely, which can reduce the dose reduction and non-specific tissue and organ toxicity
.
At the same time, in view of the reality that there is no standard treatment plan for brain metastases from breast cancer in China, Shengnuoji will make every effort to promote the world's first class 1 new drug that uses peptide coupling to specifically deliver paclitaxel to the brain - SNG1005 Phase II/III and Phase I II Phase two clinical trials, and the product will be approved and marketed in China as soon as possible
.
On this basis, accelerate the progress of SNG201, SNG202, SNG203, SNG2001, SNG2002, SNG2003 and other projects, and strive to find solutions more suitable for Chinese patients
.
2-class innovative traditional Chinese medicine icariin (acoladine) soft of Beijing Shennuoji Pharmaceutical Technology Co.
, Ltd.
, a subsidiary of Beijing Shengnuoji Pharmaceutical Technology Co.
, Ltd.
Application for marketing registration of capsules
.
Icariin (Acoradine) Soft Capsule is a first-in-class Chinese medicine (first-in-class) developed by Beijing Shengnuoji Pharmaceutical Technology Co.
, Ltd.
for 15 years.
Professor Qin Shukui from the Qinhuai Medical District of the Eastern Theater General Hospital is the chief expert, through a randomized controlled, double-blind and double-simulation, national multi-center phase III clinical study of superiority
.
Clinical trial data show that: icariin (acoladine) soft capsules are suitable for patients with unresectable hepatocellular carcinoma who are not suitable or who refuse to receive standard treatment and who have not received systemic systemic therapy before.
Markers meet at least two of the following detection indicators: AFP≥400 ng/mL; TNF-α<2.
5 pg/mL; IFN-γ≥7.
0 pg/mL
.
PART.
01 patients have significant survival benefit, and the safety advantage is outstanding.
Icariin (Acoradine) soft capsule is the first small-molecule immunomodulator (content 98.
0-102.
0%) derived from the traditional Chinese medicine Epimedium
.
Studies have shown that acoradine inhibits the TLR-MyD88-IKK-NF-κB inflammatory pathway by directly binding to MyD88/IKKα, thereby reducing the production of inflammatory factors such as TNF-α and IL-6, and down-regulating the IL6-JAK2-STAT3 pathway
.
At the same time, it directly binds to IKKα to inhibit the activation of TNF-α on the IKK-NF-κB signaling pathway, thereby inhibiting the expression of PD-L1 and the effect of MDSC, activating IFN-γ positive CD8+ T cells, and exerting anti-tumor effect.
.
Its phase II clinical study showed that acridine can improve overall survival (OS) in patients with advanced hepatocellular carcinoma compared with historical control data, and overall survival was significantly correlated with immune biomarkers
.
Phase III clinical trials use composite biomarkers and adaptive enrichment designs
.
Among the 283 patients enrolled in the whole population, the enriched population (that is, patients whose peripheral blood composite markers meet at least two of the following detection indicators: AFP≥400 ng/mL; TNF-α<2.
5 pg/mL; IFN-γ≥7.
0 pg /mL)) 71 cases
.
Among them, there were 33 cases in the acoladine group and 38 cases in the cinobufacin group
.
After analyzing 20 risk factors associated with severe baseline and poor prognosis, such as BCLC stage C, tumor burden accounted for 50%, platelet count 75x109/L, AFP≥400ng/mL, etc.
In the enriched population, the proportions of subjects meeting all three risk factors were 94% and 97%, respectively
.
The median follow-up time of the study was 8.
1 months, and the median OS of the enriched population in the acoladine group and cinobufacini group was 13.
54 and 6.
87 months, respectively (hazard ratio HR=0.
43, 95%CI 0.
23-0.
82, p=0.
0092 ), investigator-assessed median TTP was 3.
65 vs 1.
84 months (hazard ratio HR=0.
67, 95%CI, 0.
36-1.
22, p=0.
1665), DCR was 48.
5% (30.
8%-66.
5%) and 26.
3 % (13.
4% - 43.
1%), p=0.
0712
.
Median time to deterioration (mTTD) based on quality of life (QOL) scale assessment was 7.
3 vs 2.
8 months in the enriched population of the two groups (HR=0.
43, 95%CI 0.
19-0.
98, p=0.
039) , 7.
3 and 3.
7 months in the full population, respectively (HR=0.
66, 95% CI 0.
44-0.
98, p=0.
037)
.
In terms of safety, the acoladine group had a lower incidence of various adverse events compared with the cinobufacini group: the incidence of adverse events related to the study drug in the whole population was 61.
7%: 78.
7%, grade ≥3.
The incidence of adverse events related to the study drug was 12.
1%: 26.
2%, the incidence of adverse events related to the study drug leading to permanent discontinuation was 0.
7%: 2.
1%, the incidence of adverse events related to the study drug leading to dose adjustment The incidence of serious adverse events related to the study drug was 4.
3%:17.
7% and 0:5.
7%
.
The safety results observed in the enriched population were similar to those in the full population
.
PART.
02 is close to the needs of Chinese patients and fills the precise treatment plan for advanced hepatocellular carcinoma.
In recent years, many breakthroughs have been made in clinical research on systemic treatment drugs for hepatocellular carcinoma, including targeted and immunotherapy.
However, in international and domestic clinical practice of advanced hepatocellular carcinoma, the prognosis There are currently no standard or optimal first-line treatment options for patients with poor advanced hepatocellular carcinoma
.
Most patients with HCC in China are diagnosed with advanced liver cancer, and more than 80% have a history of HBV infection.
Relative to some patients with advanced HCC, the constitution is poor and often accompanied by multiple underlying diseases and prognostic risk factors, such as severe liver function damage.
, the immune function is abnormal, the side effects of the existing first-line treatment are generally large, and the tolerance is poor
.
These patients have high bilirubin, low platelets, and poor liver function in clinical treatment.
When using existing chemotherapy and targeted drug therapy, they generally have poor prognosis and high toxic and side effects (grade 3 and 4 adverse effects).
response)
.
At present, in domestic clinical practice, some doctors use traditional Chinese medicine preparations as alternative medicines, but most of these medicines lack the support of high-quality, randomized, controlled, and multi-center clinical trials
.
"Icaritin (Acoradine) Soft Capsules, as a small molecule immunomodulatory drug, can significantly prolong the overall survival and improve the quality of life of the advanced hepatocellular carcinoma-enriched population with heavier baseline and poor prognosis, and Has significant clinical safety advantages
.
"Professor Qin Shukui pointed out that the icariin (acoladine) research plan fully draws on the latest international clinical enrichment design guidelines and the successful experience of published enrichment design (REACH2) clinical research, combined with the clinical characteristics of Chinese hepatitis B virus.
The precise enrichment design of the heterogeneous characteristic markers in patients with poor prognosis has promoted the precise clinical research and treatment exploration of advanced hepatocellular carcinoma; Advanced hepatocellular carcinoma patients provide new solutions that are closer to Chinese patients, and also open up new directions for the inheritance, innovation and exploration of local innovative drugs, especially traditional Chinese
medicine
.
Combining with biotechnology, a biopharmaceutical enterprise focusing on drug research and development in the field of tumor treatment, Shengnuoji has always been the focus of attention in the field of pharmaceutical investment.
Lin Capital, Tus Venture Capital, Shenzhen Venture Capital, Legend Capital, China Life and other venture capital companies have invested more than 1 billion yuan in total
.
With continuous high-intensity investment, the scientific research system of Shengnuoji has been increasingly improved
.
The company has passed the Introduced talents and team building, developed and constructed natural small molecule drug development platform, synthetic small molecule drug research and development platform, biological macromolecule new drug research and development platform and estrogen receptor target research and development platform; has a number of national-level specially-appointed experts, provincial (city) )-level specially-appointed experts, some core technicians have more than 20 years of experience in new drug research and development, and have the experience of leading or participating in the successful launch of multiple new drugs, providing support for high-quality innovative research and development
.
Dr.
Meng Kun, chairman of Shengnuoji Pharmaceutical, introduced , In recent years, the company's research and development projects have adhered to the orientation of clinical needs, focusing on multiple malignant tumor fields with urgent clinical needs and good market prospects, and committed to the development of First in Class or Best in Class.
innovative drugs
.
At present, the company has 20 research projects with 12 products under research, covering hepatocellular carcinoma, breast cancer, non-small cell lung cancer, lymphoma and other fields.
Among them, the two indications of the research drug S NG1005 have obtained II approvals respectively.
/ Approved in Phase III and Phase III clinical trials, and the drug under investigation, fluocoradine, has been approved in Phase I clinical trials, and has also been approved for clinical trials in the United States
.
The remaining 9 drug candidates are in the preclinical research stage
.
In terms of intellectual property protection, the company has obtained 57 invention patent authorizations, including 26 authorized in mainland China and 31 authorized overseas
.
"Shengnuoji has always been based on better solving the clinical needs of domestic patients as the starting point and foothold of research and development
.
" Dr.
Meng Kun introduced that icariin (Acoradine) was used as the core structure to transform and optimize.
A chemically synthesized derivative, fluocoradine, is a brand-new tumor immunomodulatory small molecule drug, intended for use in patients with advanced hepatocellular carcinoma.
As part of the company's promotion of product internationalization, the company is developing the first The second-generation formulation (SNG168), using nano-polymer mixed micelles (PMM) as a new drug delivery system, can deliver drugs to target tissues more precisely, which can reduce the dose reduction and non-specific tissue and organ toxicity
.
At the same time, in view of the reality that there is no standard treatment plan for brain metastases from breast cancer in China, Shengnuoji will make every effort to promote the world's first class 1 new drug that uses peptide coupling to specifically deliver paclitaxel to the brain - SNG1005 Phase II/III and Phase I II Phase two clinical trials, and the product will be approved and marketed in China as soon as possible
.
On this basis, accelerate the progress of SNG201, SNG202, SNG203, SNG2001, SNG2002, SNG2003 and other projects, and strive to find solutions more suitable for Chinese patients
.