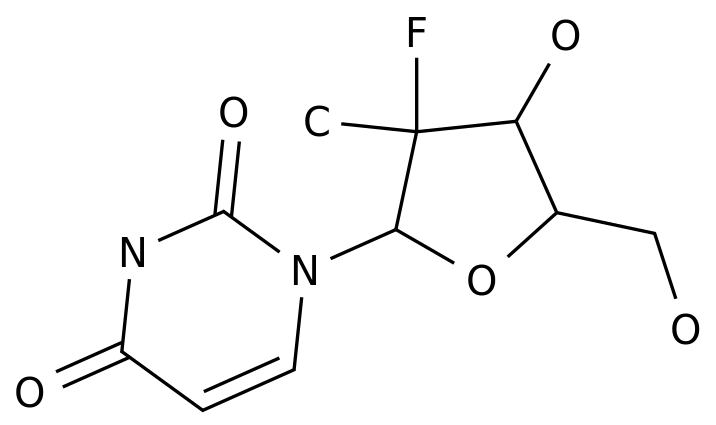
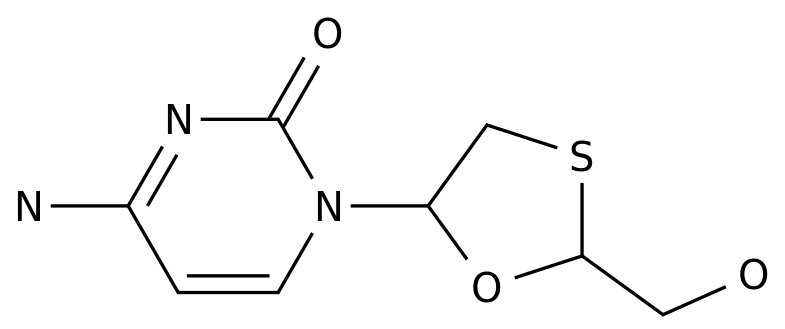
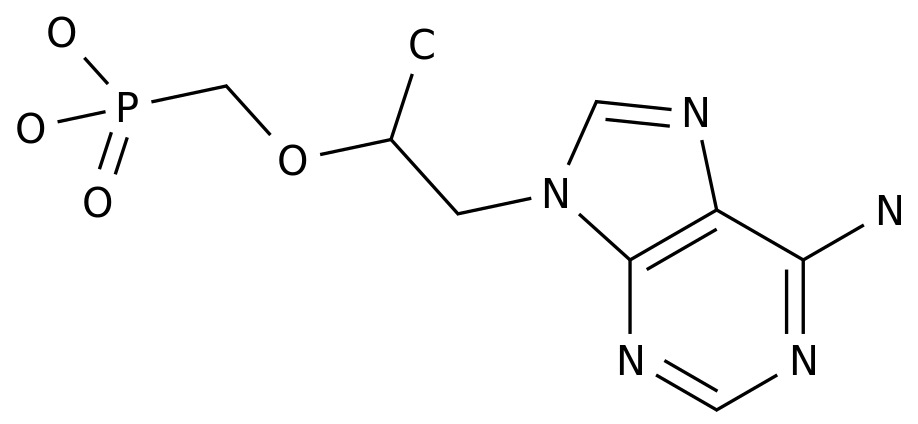
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
-
Cosmetic Ingredient
- Water Treatment Chemical
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
The production process of ethanone, 1-(2-amino-5-chlorophenyl)-2,2,2-trifluoro-, hydrochloride (1:1), is a complex and multi-step process that involves several chemical reactions and purification steps.
The final product is a white to off-white solid that is used as an intermediate in the production of various pharmaceuticals and other chemical products.
The production process of ethanone can be broken down into several steps, including:
- Preparation of the starting materials: The production process of ethanone begins with the preparation of the starting materials, which include 2-amino-5-chlorophenol and 2,2,2-trifluoroacetic acid.
These starting materials are carefully synthesized in a series of chemical reactions that involve the use of various reagents, catalysts, and conditions. - The reaction between 2-amino-5-chlorophenol and 2,2,2-trifluoroacetic acid: The next step in the production process of ethanone involves the reaction between 2-amino-5-chlorophenol and 2,2,2-trifluoroacetic acid.
This reaction is carried out in the presence of a solvent, such as water or methanol, and a catalyst, such as sodium hydroxide or potassium hydroxide.
The reaction produces the desired product, 1-(2-amino-5-chlorophenyl)-2,2,2-trifluoro-ethanone, which is then isolated and purified. - Purification of the product: The purification process involves several steps, including washing with water, followed by the addition of a solvent, such as ethyl acetate or dichloromethane, to extract the impurities.
The organic phase is then separated and dried over anhydrous sodium sulfate.
The solvent is removed under reduced pressure, and the residue is dissolved in a small amount of a solvent, such as acetonitrile or ethanol, and passed through a column packed with a suitable adsorbent, such as silica gel or alumina.
The pure product is then eluted and collected. - Hydrochloride formation: The final step in the production process of ethanone involves the formation of the hydrochloride salt.
This is achieved by dissolving the pure product in a suitable solvent, such as ethanol or methanol, and adding a suitable amount of aqueous hydrochloric acid.
The solution is then stirred for a suitable period, and the resulting precipitate is collected by filtration and dried under vacuum.
The production process of ethanone is a complex and multi-step process that requires careful planning, execution, and monitoring to ensure the production of a high-quality product.
The various steps involved in the production process of ethanone require the use of suitable equipment, such as reactors, condensers, distillation columns, and chromatography columns, and the proper handling and storage of various chemicals and reagents.
In conclusion, the production process of ethanone, 1-(2-amino-5-chlorophenyl)-2,2,2-trifluoro-, hydrochloride (1:1), is a complex and multi-step process that involves several chemical reactions and purification steps.
The final product is a white to off-white solid that is used as an intermediate in the production of various pharmaceuticals and other chemical products.
The production process requires careful planning, execution, and monitoring to ensure the production of a high-quality product.