-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
-
Cosmetic Ingredient
- Water Treatment Chemical
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
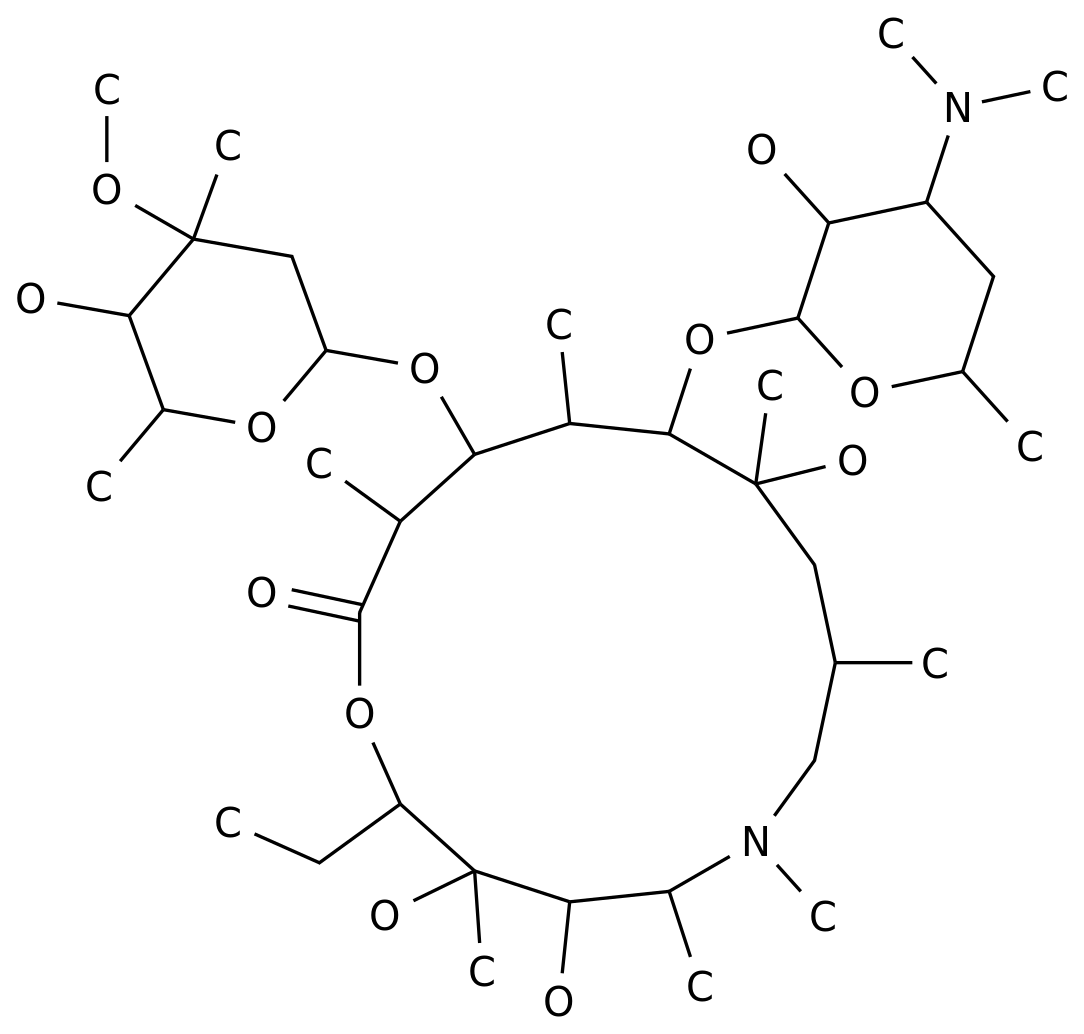
Tilmicosin phosphate is an antibiotic drug used to treat various bacterial infections.
The production process of tilmicosin phosphate involves several steps, including chemical synthesis, purification, and formulation.
In this article, we will discuss the production process of tilmicosin phosphate in detail.
Chemical Synthesis:
The chemical synthesis of tilmicosin phosphate involves several steps, including the synthesis of the basic structure of the drug and its subsequent modifications to produce the final product.
The synthesis process typically involves the use of organic chemicals and reactions, and it requires strict control over the reaction conditions to ensure the quality of the final product.
Purification:
After the synthesis process, the product is typically purified to remove any impurities that may have been introduced during the synthesis process.
Purification can be achieved through various methods, such as crystallization, chromatography, and filtration.
The purification process is critical as it ensures that the final product is free from impurities that may have adverse effects on the human body.
Formulation:
After purification, the tilmicosin phosphate is formulated into various dosage forms, such as tablets, capsules, and liquids.
Formulation involves mixing the active ingredient with other ingredients, such as excipients, preservatives, and stabilizers, to produce a final product that is stable, safe, and effective.
The formulation process is critical as it determines the shelf life, bioavailability, and efficacy of the drug.
Quality Control:
The production process of tilmicosin phosphate is subject to strict quality control measures to ensure that the final product meets the required standards for purity, potency, and efficacy.
Quality control measures include testing the raw materials, intermediate products, and finished products for various parameters, such as chemical composition, microbial contamination, and stability.
Manufacturing Scales:
The production of tilmicosin phosphate can be scaled up or down depending on the demand for the drug.
The manufacturing scale can range from small-scale laboratory synthesis to large-scale industrial production.
The scaling-up process is critical as it ensures that the production process is economically viable and that the final product is consistent in quality.
Regulatory Approval:
The production of tilmicosin phosphate requires regulatory approval from the relevant authorities, such as the Food and Drug Administration (FDA) in the United States.
The approval process involves extensive testing and evaluation of the drug to ensure that it meets the required standards for safety and efficacy.
The approval process is critical as it ensures that the drug is safe for use in humans and animals.
Advantages of Tilmicosin Phosphate:
Tilmicosin phosphate has several advantages over other antibiotic drugs, including its broad-spectrum activity, high potency, and low toxicity.
It is effective against a wide range of bacterial pathogens, including Gram-positive and Gram-negative bacteria, making it a versatile antibiotic drug.
The drug is also highly potent, with minimal toxicity to normal cells and tissues, making it safer for use in humans and animals.
Conclusion:
The production process of tilmicosin phosphate involves several steps, including chemical synthesis, purification, and formulation.
The drug is subject to strict quality control measures to ensure that it meets the required standards for purity, potency, and efficacy.
The manufacturing scale can be scaled up or down depending on the demand for the drug, and regulatory approval is required from the relevant authorities.
Tilmicosin phosphate has several advantages over other antibiotic drugs, including its broad-spectrum activity, high potency, and low toxicity, making it a versatile and safe drug for use in humans and animals.