-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
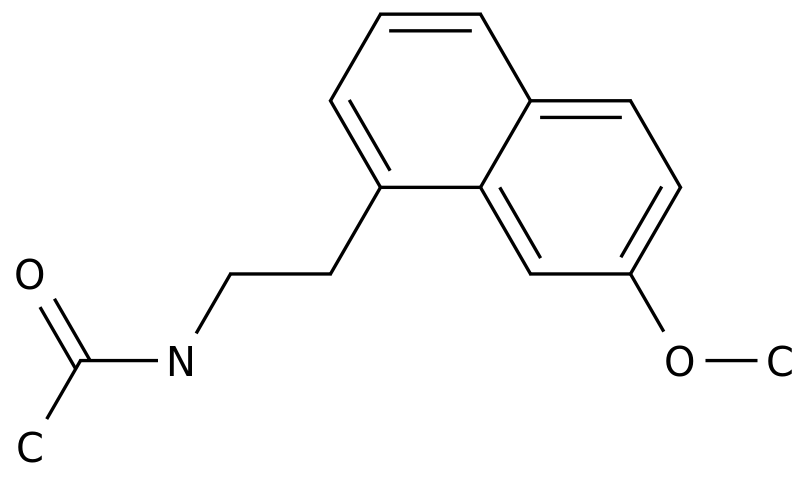
Bromocriptine mesylate is a synthetic dopamine agonist that is used in the treatment of various medical conditions such as Parkinson's disease, hyperprolactinemia, and infertility.
It is also used in research to study the physiology and pathology of the dopamine system.
In the chemical industry, the safety of bromocriptine mesylate is of utmost importance, as it is handled and used by workers who may be exposed to the chemical.
Bromocriptine mesylate is classified as a Category 2 carcinogen by the International Agency for Research on Cancer (IARC), which means that it is possibly carcinogenic to humans.
Long-term animal studies have shown an increased incidence of tumors, particularly in the liver and lung, in animals treated with bromocriptine mesylate.
However, it is important to note that these studies were conducted using high doses of the chemical, and the relevance to human exposure is uncertain.
Studies in humans have shown that bromocriptine mesylate can cause a range of adverse effects, including nausea, dizziness, headache, and hypotension.
These effects are generally mild and occur at high doses or with long-term use.
The safety of bromocriptine mesylate has been evaluated in clinical trials involving thousands of patients, and the overall incidence of adverse effects was low.
In terms of environmental safety, bromocriptine mesylate is not highly soluble in water and is not expected to have a significant impact on aquatic ecosystems.
However, it is highly soluble in fat, and it is possible that it could accumulate in animal tissues, including those of wildlife.
The handling of bromocriptine mesylate should be done with caution, and appropriate protective measures should be taken to minimize exposure.
This includes wearing appropriate personal protective equipment, such as gloves and masks, and following proper safety procedures and guidelines.
Exposure to bromocriptine mesylate should be limited as much as possible, and workers should be trained on the potential health hazards associated with the chemical.
It is important to note that the safety of bromocriptine mesylate can be affected by a range of factors, including the duration and intensity of exposure, the individual's health status, and other environmental and lifestyle factors.
Therefore, regular monitoring and assessment of the safety of bromocriptine mesylate is necessary to ensure that it continues to be safe for use in the chemical industry.
In conclusion, bromocriptine mesylate is a synthetic dopamine agonist that is used in the treatment of various medical conditions and in research.
While it is classified as a Category 2 carcinogen by the IARC, the relevance to human exposure is uncertain.
Long-term animal studies have shown an increased incidence of tumors, particularly in the liver and lung, in animals treated with bromocriptine mesylate.
Studies in humans have shown that bromocriptine mesylate can cause a range of adverse effects, including nausea, dizziness, headache, and hypotension, which are generally mild and occur at high doses or with long-term use.
The safety of bromocriptine mesylate has been evaluated in clinical trials involving thousands of patients, and the overall incidence of adverse effects was low.
The handling of bromocriptine mesylate should be done with caution, and appropriate protective measures should be taken to minimize exposure.
Regular monitoring and assessment of the safety of bromocriptine mesylate is necessary to ensure that it continues to be safe for use in the chemical industry.