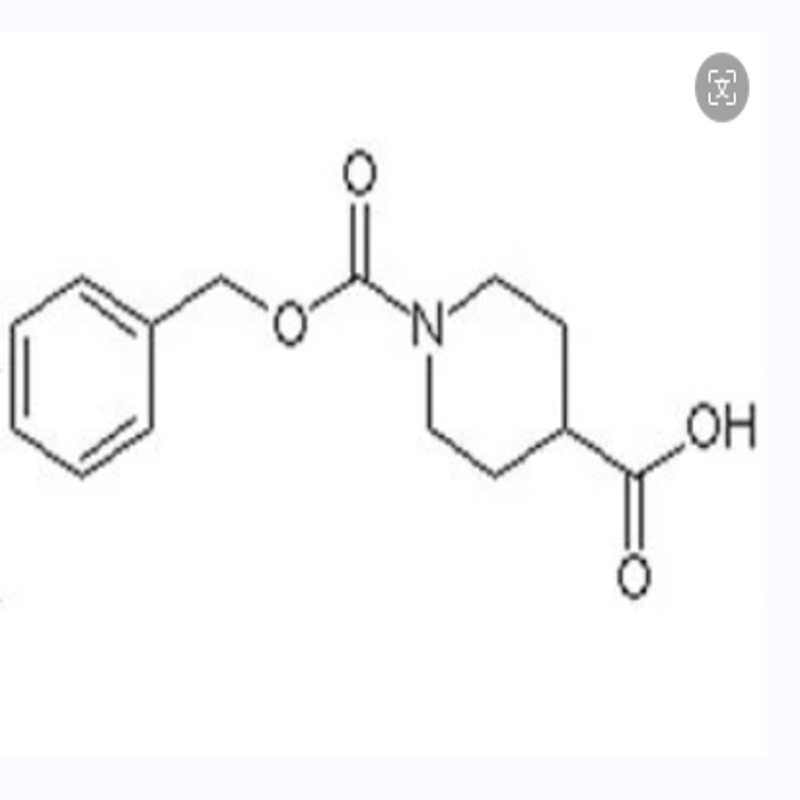
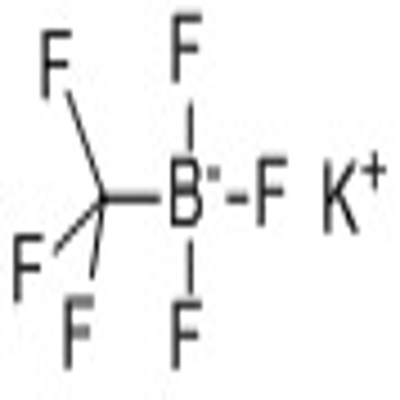
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
On October 30, Jiangsu Osaikang Class 3 generic drug "apple acid cabotinib tablets" submitted applications for listing and were accepted, for the second domestic newspaper listing.
, Howson debuted on September 24.
Cabozantinib, developed by Exelixis and Ipsos Pharmaceuticals, is a multi-target small molecule tyrosine kinase inhibitor that targets met, VEGFR1/2/3, ROS1, RET, AXL, NTRK, KIT, etc.
was first approved by the FDA in November 2012 for the treatment of progressive, metastatic thyroid myelin-like cancer (MTC).
April 2016, the FDA approved cabotinib for the first time for second-line treatment in patients with advanced renal cell carcinoma (RCC) who had previously received anti-angiogenesis treatment, based on the results of the Cabotinib VS Evimox clinical trial (research code METEOR).
December 2017, the FDA further approved Cabotinib for first-line treatment in patients with advanced renal cell carcinoma (RCC) based on the results of the Cabotinib VS Schoiniene Clinical Trial (research code CABOSUN).
January 2019, the FDA officially approved Cabotinib for second-line treatment in patients with advanced liver cancer, based on the clinical study code name CELESTIAL.
Currently, Cabotinib has been clinically and has shown good therapeutic results in a variety of solid tumors, including thyroid myelin, kidney, non-small cell lung cancer, liver cancer, prostate cancer, breast cancer, ovarian cancer, bowel cancer, and so on.
because of its wide-ranging effectiveness for a variety of cancers, cabotinib is known as the "golden oil" in targeted drugs and has broad-spectrum cancer resistance.
, however, the original research has not been listed in China, according to insight database, Cabozantinib (XL184) and Atezolizumab drugs for advanced hepatocellular carcinoma clinical has entered phase 3.
Although the original research has not been listed, many domestic generic drug companies have long since begun to lay out, Insight database shows that at present, in addition to Howson and Osaikang, Zheng Tianqing, Jiangsu Weikel, Xiansheng Dongyuan has been doing biological equivalent testing.
from: Insight Database ()