-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
-
Cosmetic Ingredient
- Water Treatment Chemical
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
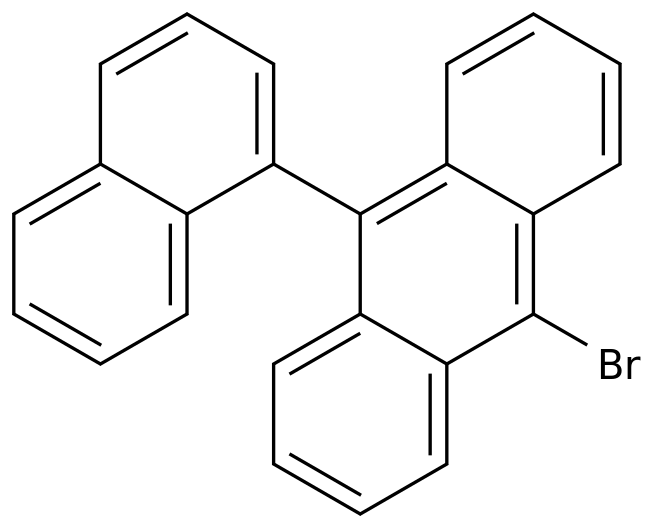
3-Bromo-9,10-phenanthrenedione is an important intermediate in the synthesis of various chemicals and pharmaceuticals.
It is also used as a reagent in organic synthesis.
The synthesis of 3-bromo-9,10-phenanthrenedione can be achieved through different routes, and this article will discuss some of the most common synthetic routes to this compound.
- electrophilic substitution
One of the most common methods of synthesizing 3-bromo-9,10-phenanthrenedione is through electrophilic substitution, which involves the replacement of a functional group in an aromatic ring with a different functional group.
In this case, the bromine atom is added to the benzene ring of 3-bromo-9,10-phenanthrenedione, resulting in the formation of a new functional group.
- Halogenation
Another method of synthesizing 3-bromo-9,10-phenanthrenedione is through halogenation, which involves the substitution of a halogen atom for a hydrogen atom in an aromatic ring.
This method is less commonly used than electrophilic substitution, but it is still a viable synthetic route.
- Ring-closing metathesis
3-bromo-9,10-phenanthrenedione can also be synthesized through ring-closing metathesis, which is a catalytic process that involves the reaction of two substrates to form a cyclic compound.
In this case, the reaction involves the reaction of two aromatic compounds with a bromine atom in the presence of a metal catalyst.
- Direct bromination
In the direct bromination method, 3-bromo-9,10-phenanthrenedione is synthesized by the direct reaction of benzene with bromine in the presence of a solvent such as carbon tetrachloride.
This method is less commonly used due to the high reactivity of benzene and the risk of explosion.
- Hydrolysis of bromoacetophenone
3-bromo-9,10-phenanthrenedione can also be synthesized by the hydrolysis of bromoacetophenone, which is a derivative of acetophenone.
In this method, bromoacetophenone is treated with water, and the resulting product is then purified to obtain 3-bromo-9,10-phenanthrenedione.
- Direct halogenation
In the direct halogenation method, 3-bromo-9,10-phenanthrenedione is synthesized by the direct reaction of 3-bromo-9-fluorenone with sodium hydroxide in the presence of water.
This method is a relatively simple and efficient way to synthesize 3-bromo-9,10-phenanthrenedione.
In conclusion, 3-bromo-9,10-phenanthrenedione can be synthesized through several methods, including electrophilic substitution, halogenation, ring-closing metathesis, direct bromination, hydrolysis of bromoacetophenone, and direct halogenation.
The choice of synthetic route depends on the availability of starting materials and the desired yield and purity of the final product.
Regardless of the method used, the synthesis of 3-bromo-9,10-phenanthrenedione is an important step in the production of various chemicals and pharmaceuticals, and it remains an active area of research in the chemical industry.