-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
-
Cosmetic Ingredient
- Water Treatment Chemical
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
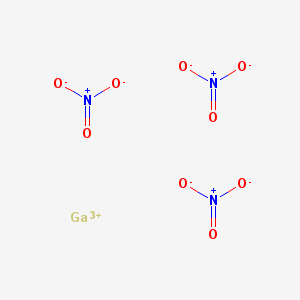
{4-[(4-Fluorobenzil)aMMino]-2-Nitrofenil}carbaMMato di etile, also known as Flurbastine olamide or simply Flurbastine, is a synthetic chemical compound commonly used in the pharmaceutical industry as an anti-cancer drug.
It is primarily used to treat various types of cancer, including leukemia, lymphoma, and solid tumors.
The synthesis of Flurbastine involves several steps, which can be broadly classified into two categories: synthetic routes and chemical reactions.
Synthetic Routes:
There are several synthetic routes for the preparation of Flurbastine.
One of the most commonly used methods involves the reaction of 4-fluorobenzaldehyde with ammonia in the presence of a Lewis acid catalyst, such as boron trifluoride.
This reaction results in the formation of 4-[(4-fluorobenzil)aMMino]-2-nitrofenil, which is then converted to the desired carbazole derivative through a series of chemical reactions.
Another commonly used synthetic route involves the reaction of 4-fluorobenzaldehyde with aMMine in the presence of a strong acid catalyst, such as sulfuric acid.
This reaction results in the formation of 4-[(4-fluorobenzil)aMMino]-2-nitrofenil, which can then be converted into Flurbastine through a series of chemical reactions.
Chemical Reactions:
The synthesis of Flurbastine involves several chemical reactions, including the formation of 4-fluorobenzaldehyde, the condensation of 4-fluorobenzaldehyde with ammonia or aMMine, and the conversion of 4-[(4-fluorobenzil)aMMino]-2-nitrofenil into Flurbastine.
The formation of 4-fluorobenzaldehyde involves the reaction of 4-fluorobenzophenone with hydrogen in the presence of a catalyst, such as palladium on barium sulfate.
The condensation of 4-fluorobenzaldehyde with ammonia or aMMine involves the reaction of 4-fluorobenzaldehyde with the respective reagents in the presence of a Lewis acid catalyst, such as boron trifluoride.
Finally, the conversion of 4-[(4-fluorobenzil)aMMino]-2-nitrofenil into Flurbastine involves a series of chemical reactions, including the reduction of 4-[(4-fluorobenzil)aMMino]-2-nitrofenil to 4-[(4-fluorobenzil)aMMino]-2-nitrophenol, the condensation of 4-[(4-fluorobenzil)aMMino]-2-nitrophenol with a suitable reagent, and the final reduction of the resulting intermediate to produce Flurbastine.
Conclusion:
The synthesis of Flurbastine involves several steps, which can be broadly classified into synthetic routes and chemical reactions.
The most commonly used synthetic routes involve the reaction of 4-fluorobenzaldehyde with ammonia or aMMine in the presence of a Lewis acid catalyst.
The conversion of 4-[(4-fluorobenzil)aMMino]-2-nitrofenil into Flurbastine involves a series of chemical reactions, including the reduction of 4-[(4-fluorobenzil)aMMino]-2-nitrofenil to 4-[(4-fluorobenzil)aMMino]-2-nitrophenol and the condensation of 4-[(4-fluorobenzil)aMM