-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
-
Cosmetic Ingredient
- Water Treatment Chemical
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
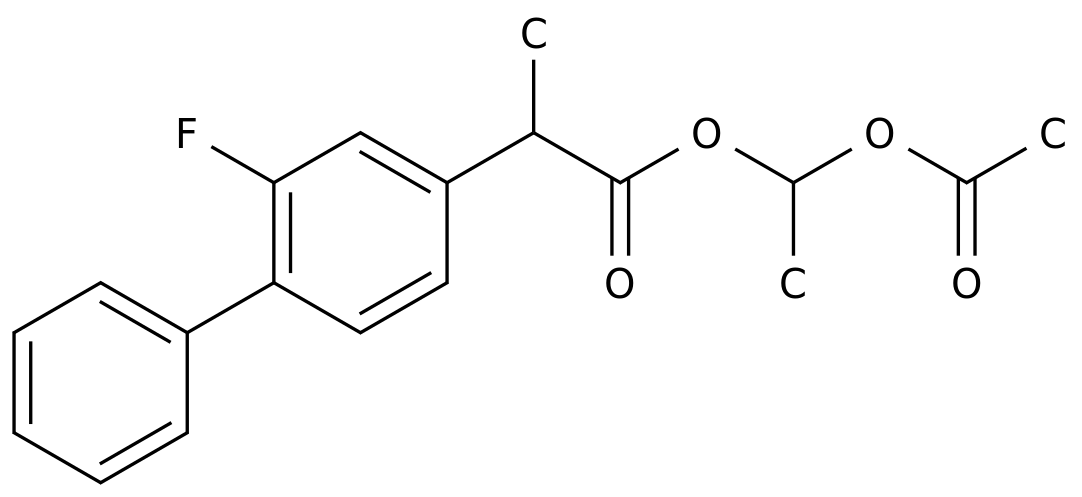
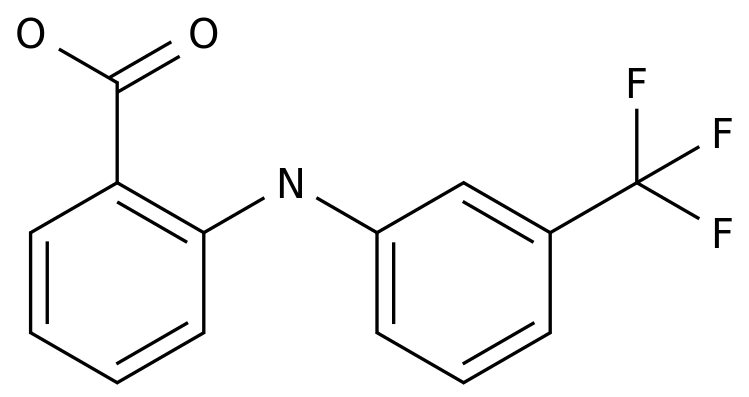
Flunixin meglumine is an antipyretic and analgesic drug that is commonly used in the treatment of fever and pain.
It is a synthetic drug that is derived from salicylic acid, which is a natural compound that is found in certain plants, such as willow trees.
The synthetic routes of flunixin meglumine can be broadly classified into two categories: chemical synthesis and biotechnological synthesis.
Chemical synthesis involves the use of chemical reactions to synthesize the drug from its constituent chemicals.
The most common chemical synthesis route for flunixin meglumine involves the synthesis of salicylic acid, which is then converted into flunixin meglumine using a series of chemical reactions.
The chemical synthesis route typically involves the following steps:
- Synthesis of salicylic acid: The first step in the synthesis of flunixin meglumine is the synthesis of salicylic acid.
This is typically accomplished by the hydrolysis of sodium salicylate, which is a chemical that is readily obtained from willow trees. - Acid-base extraction: After the synthesis of salicylic acid, it is extracted from the reaction mixture using a basic solution, such as sodium hydroxide.
- Cyclization: The next step is the cyclization of salicylic acid to form 2-aminobenzamide, which is a key intermediate in the synthesis of flunixin meglumine.
- Amination: 2-aminobenzamide is then treated with an aqueous solution of ammonia to form 2-aminobenzamide hydrochloride.
- Deacylation: The next step is the deacylation of 2-aminobenzamide hydrochloride, which involves the removal of the benzamide group.
- Amination: The resulting compound is then treated with an aqueous solution of ammonia to form N-methyl-N-(2-hydroxyethyl) amino acid, which is a key intermediate in the synthesis of flunixin meglumine.
- Decarboxylation: The final step is the decarboxylation of N-methyl-N-(2-hydroxyethyl) amino acid, which involves the removal of carbon dioxide to form flunixin meglumine.
This chemical synthesis route typically requires the use of several chemical reagents and solvents, and can be expensive and time-consuming.
In addition, the use of chemical reagents can be hazardous, and proper safety protocols must be followed to prevent accidents.
Biotechnological synthesis involves the use of living organisms, such as bacteria or yeast, to synthesize the drug.
The biotechnological synthesis of flunixin meglumine typically involves the following steps:
- Selection of the host organism: The first step in the biotechnological synthesis of flunixin meglumine is the selection of the host organism.
This is typically accomplished by screening a variety of organisms to identify the one that is most efficient at synthesizing the drug. - Construction of the expression vector: After the selection of the host organism, the next step is the construction of the expression vector, which is a genetic construct that contains the gene for the enzyme that is responsible for the synthesis of flunixin meglumine.
- Introduction of the expression vector into the host organism: The expression vector is then introduced into the host organism using a technique called transformation.
This involves the introduction of the expression vector into the cells of the host organism, where it is expressed and used to synthesize the drug. - Fermentation: The final step is the fermentation of the host organism, which involves the growth of the cells and the synthesis of flunixin meglumine.
The drug is then extracted