-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
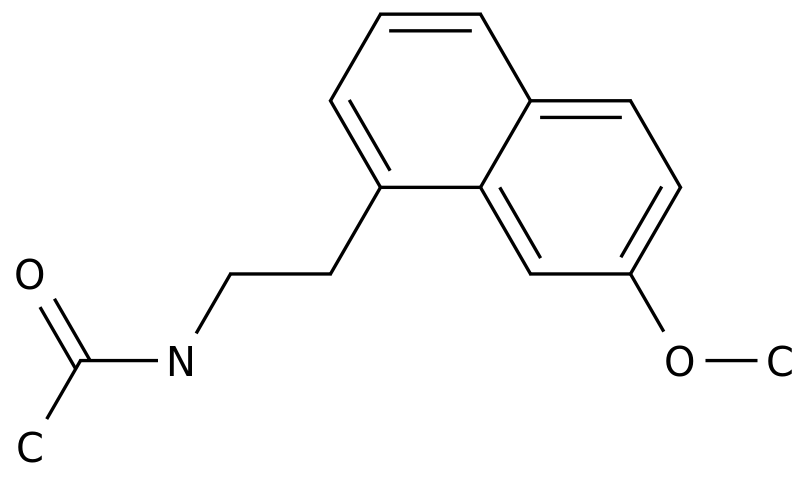
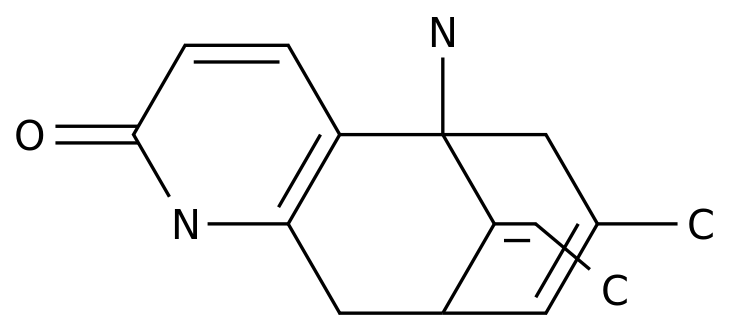
The Synthetic Route of Metapramine: An Overview of the Process in the Chemical Industry
Metapramine is a drug used in the treatment of depression and other related disorders.
It is classified as a secondary amine derivative and belongs to a group of medications known as tricyclic antidepressants.
In the chemical industry, the synthetic route of Metapramine is a complex process that involves several chemical reactions.
The synthesis of Metapramine typically starts with the reaction of aniline, a chemical compound derived from coal tar, with an activated aromatic compound.
The reaction produces a derivative of aniline known as N-methylaniline, which is then reacted with an electrophile, such as chloride or bromide, to form a new compound.
This compound undergoes further chemical reactions, such as hydrogenation and alkylation, to form the final product, Metapramine.
The synthetic route of Metapramine is not a straightforward process and requires careful control of the reaction conditions to ensure the highest possible yield of the desired product.
This requires the use of specialized equipment and the employment of skilled operators who have a thorough understanding of the process.
The synthesis of Metapramine is a complex process that involves several chemical reactions.
The key to successful synthesis is the control of the reaction conditions, including temperature, pressure, and the use of catalysts, to ensure the highest possible yield of the desired product.
Advances in Synthetic Techniques
In recent years, there have been significant advances in the synthetic techniques used to produce Metapramine.
One of the key advances has been the development of new catalytic systems that allow for more efficient and cost-effective synthesis of the drug.
Another major advance has been the development of new methods for the synthesis of Metapramine, such as the use of microwave irradiation and ultrasound.
These methods allow for faster reaction times and higher yields of the desired product, while also reducing the amount of waste generated during the synthetic process.
Challenges in the Synthesis of Metapramine
Despite the advances in synthetic techniques, the synthesis of Metapramine still presents several challenges.
One of the most significant challenges is the need for strict control of the reaction conditions to ensure the highest possible yield of the desired product.
Another significant challenge is the high energy input required for the synthesis of Metapramine, as well as the need for the use of hazardous reagents and solvents.
These factors can make the synthesis of Metapramine both costly and environmentally unfriendly.
Future Directions in Synthetic Route for Metapramine
Despite the challenges, there is ongoing research in the chemical industry to find more efficient and environmentally friendly methods for the synthesis of Metapramine.
One of the areas of focus is the development of new methods for the synthesis of the drug using sustainable and renewable feedstocks.
Another area of focus is the development of new catalytic systems that can be used to replace the hazardous reagents and solvents currently used in the synthesis of Metapramine.
These efforts aim to make the production of the drug more environmentally friendly and cost-effective.
Conclusion
The synthetic route of Metapramine is a complex process that requires careful control of the reaction conditions to ensure the highest possible yield of the desired product.
Despite the challenges, there is ongoing research in the chemical industry to find more efficient and environmentally friendly methods for the synthesis of Metapramine.
These efforts aim to make the production of the drug more sustainable and cost-effective, while also reducing its environmental impact.