-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
-
Cosmetic Ingredient
- Water Treatment Chemical
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
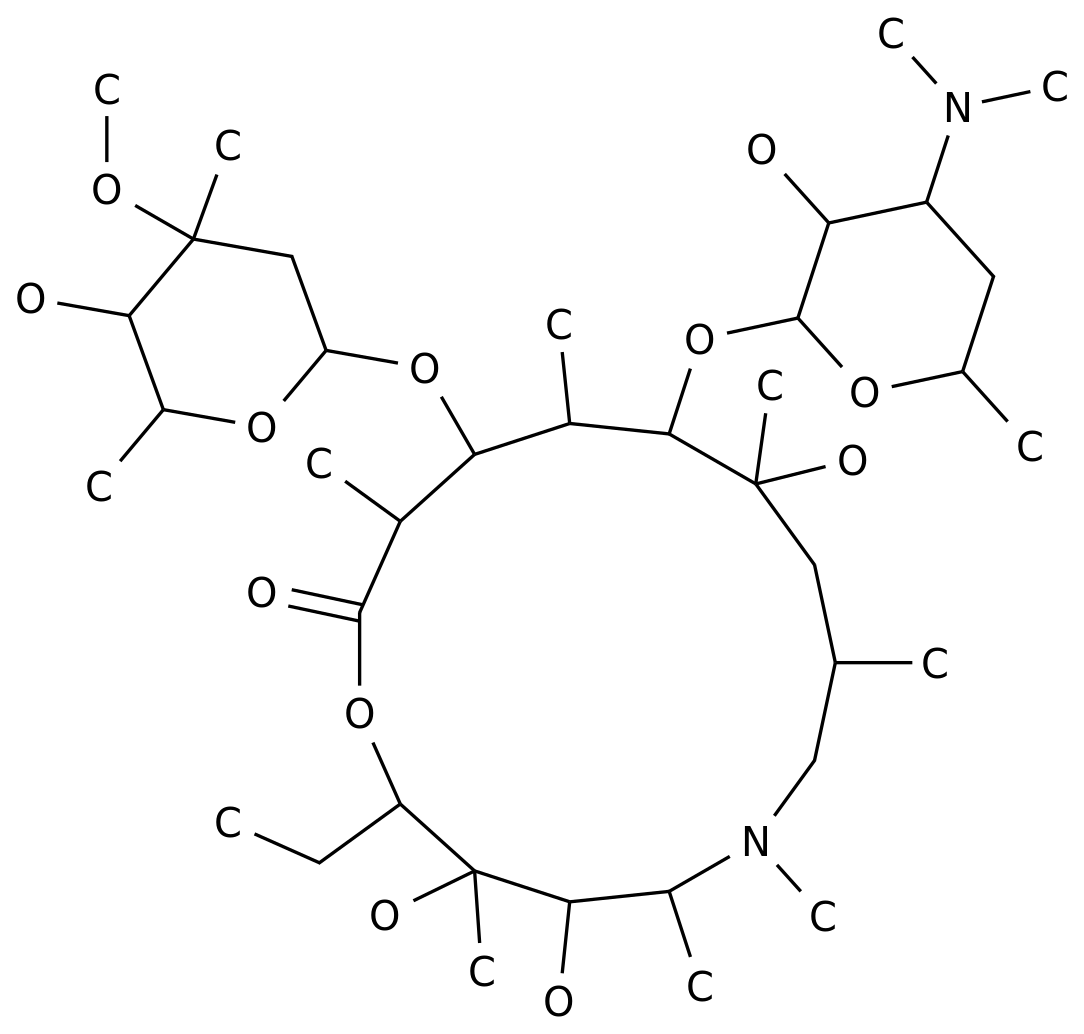
Streptomycin sulfate is an antibiotic that is commonly used to treat a variety of bacterial infections.
It is produced through a number of different synthetic routes, each with its own advantages and disadvantages.
In this article, we will explore the different synthetic routes of streptomycin sulfate and their significance in the chemical industry.
The first synthetic route for streptomycin sulfate was developed in the 1940s by the American chemist Albert Schotte.
This route involved the synthesis of the molecule's basic structure, known as the streptomycin skeleton, through a series of chemical reactions.
The streptomycin skeleton was then modified with a sulfuric acid group to create streptomycin sulfate.
One of the main advantages of this route is that it is relatively simple and straightforward, making it a popular choice for large-scale production.
However, it does require the use of hazardous chemicals, such as sulfuric acid, which can make it less safe for workers to handle.
Another synthetic route for streptomycin sulfate involves using a natural product called nocardamide as a starting material.
This route was developed in the 1960s by the Japanese chemist K.
Kimata.
It is considered to be more environmentally friendly than the Schotte route, as it does not require the use of hazardous chemicals.
The Kimata route involves a series of chemical reactions that transform nocardamide into streptomycin sulfate.
One of the main advantages of this route is that it is more efficient than the Schotte route, as it produces higher yields of the final product.
However, it does require the use of specialized equipment and a higher level of expertise, making it less accessible for small-scale production.
Another synthetic route for streptomycin sulfate was developed in the 1990s by the German chemist W.
Zink.
This route involves the use of a naturally occurring bacteria called Streptomyces griseus as a starting material.
This route is considered to be the most environmentally friendly of all the synthetic routes, as it does not require the use of any hazardous chemicals.
The Zink route involves a series of biotechnological steps, in which the bacteria are manipulated to produce the streptomycin sulfate molecule.
One of the main advantages of this route is that it is highly efficient, as it produces high yields of the final product with minimal waste.
However, it does require specialized equipment and a high level of technical expertise, making it less accessible for small-scale production.
In conclusion, there are several different synthetic routes for streptomycin sulfate, each with its own advantages and disadvantages.
While the Schotte and Kimata routes are more commonly used in industry due to their simplicity and efficiency, the Zink route is considered to be the most environmentally friendly.
As the demand for antibiotics continues to grow, it is likely that new and more efficient synthetic routes for streptomycin sulfate will be developed.