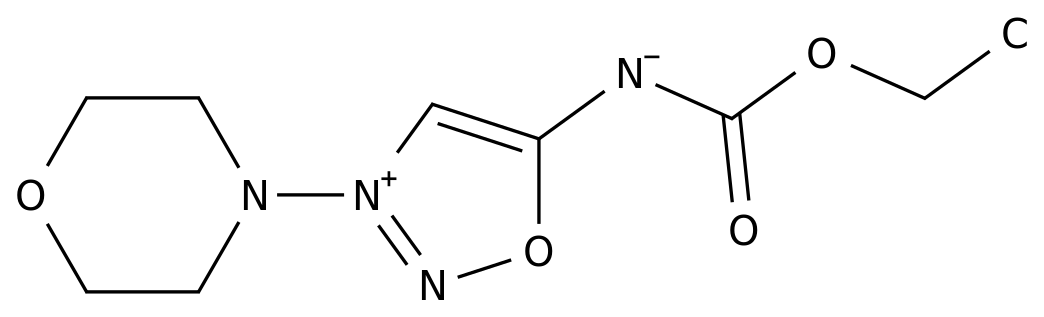
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
-
Cosmetic Ingredient
- Water Treatment Chemical
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
Tirofiban hydrochloride monohydrate is an important pharmaceutical drug that is used for the treatment of acute coronary syndrome.
It is a platelet glycoprotein IIb/IIIa inhibitor that works by inhibiting the binding of platelet glycoprotein IIb/IIIa with fibrinogen, which results in a reduction in the formation of thrombus or blood clots.
The synthesis of Tirofiban hydrochloride monohydrate involves several steps and can be achieved through different synthetic routes.
One of the common synthetic routes for Tirofiban hydrochloride monohydrate is through the coupling of a diversely substituted benzene sulfonamide with a key intermediate, which is a protected amino acid.
This reaction is performed in the presence of a base, such as sodium hydroxide, and a solvent, such as dimethylformamide, to provide the desired product.
The protected amino acid is then deprotected using hydrochloric acid, and the resulting product is further purified using column chromatography.
Another synthetic route for Tirofiban hydrochloride monohydrate involves the condensation of an aromatic aldehyde with a substituted salicylamide in the presence of a condensing agent, such as dicyclohexylcarbodiimide.
This reaction is typically performed in a polar solvent, such as dimethylformamide, and the resulting product is then purified using column chromatography.
An alternate synthetic route for Tirofiban hydrochloride monohydrate involves the reaction of a substituted aromatic primary amine with an aromatic aldehyde in the presence of a reducing agent, such as sodium borohydride.
This reaction is typically performed in a polar solvent, such as dimethylformamide, and the resulting product is then purified using column chromatography.
Finally, Tirofiban hydrochloride monohydrate can also be synthesized through a total synthesis route that involves the synthesis of the entire molecule from basic building blocks, such as aniline and piperidine.
This synthesis route typically involves a series of reactions, including nitration, halogenation, diazo coupling, and finally, the condensation of the various components to form the final product.
One of the main advantages of the synthetic routes for Tirofiban hydrochloride monohydrate is that they allow for the production of the drug in large quantities at a relatively low cost.
Additionally, the synthetic routes also allow for the modification of the chemical structure of the drug, which can lead to the development of new and improved versions of Tirofiban hydrochloride monohydrate.
The safety and efficacy of Tirofiban hydrochloride monohydrate have been established through extensive clinical trials and have been proven to be effective in the treatment of acute coronary syndrome.
Additionally, the drug has been found to be safe and well-tolerated in patients, with few reported side effects.
In conclusion, Tirofiban hydrochloride monohydrate is an important pharmaceutical drug that is widely used for the treatment of acute coronary syndrome.
The synthetic routes for the production of Tirofiban hydrochloride monohydrate allow for the production of the drug in large quantities at a relatively low cost and also allow for the development of new and improved versions of the drug.
The safety and efficacy of Tirofiban hydrochloride monohydrate have been established through extensive clinical trials, and the drug has been found to be safe and well-tolerated in patients.