-
Categories
-
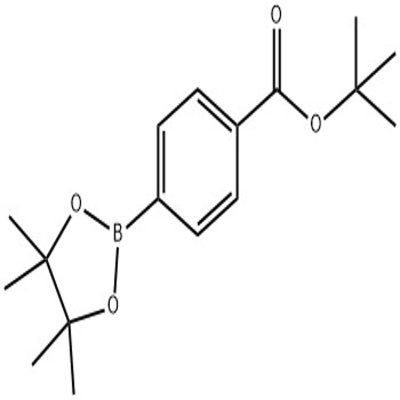
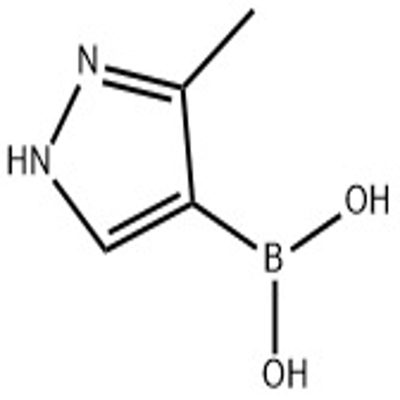
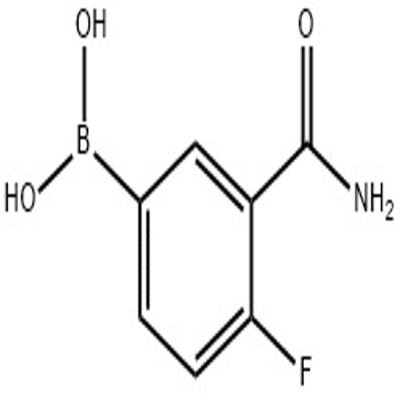
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
Text . . In May 2020, the U.S. Pharmacopeia Convention (USPC) held a web-based meeting to review its central role in advancing public health and to evaluate its response to the urgent needs of the COVID-19 crisis.
also adopted 15 resolutions aimed at setting strategic priorities for the next five-year cycle.
the USP Convention is held every five years, bringing together representatives of voting member organizations (there are nearly 500) to discuss and vote on strategies to guide the USP's operations over the next five-year cycle.
the USPC conference, which coincides with the 200th anniversary of the USP this year, is only held as a remote meeting due to the COVID-19 outbreak.
meeting, USP Ceo Ron Piervincenzi detailed USP's progress in meeting the priorities set out in the 11 resolutions adopted at the 2015 USPC conference, which addressed key challenges at the time, including focusing on modernization, working more closely with USP stakeholders, and ensuring that USP growth and resource management and direction were more consistent.
He also described usP's achievements in working with the FDA over the past five years, including creating a pending monograph program, bringing together stakeholders to help mitigate opioid misuse and abuse through labeling and packaging standards, prioritizing quality standards for OTC drugs, such as acetaminophen-containing products and testing for key impurities, and supporting the FDA's Drug Competition Action Plan (DCAP).
that for the monograph to be most effective, it must include the latest analytical methods and testing requirements that reflect the state of the industry.
he reported on USP's progress in modernizing its methodology among its more than 6,800 standards to promote common quality goals for global drugmakers, eliminate outdated technologies, provide the basis for manufacturing innovation, and ensure the most basic impurities are identified and removed from processes.
usP leadership described the 2020-2025 strategy before this year's meeting, the USP Board of Directors approved the strategy for the 2020-2025 cycle.
Piervincenzi explains how the new "impact strategy" will guide USP in its public health mission over the next five years and beyond.
In developing the strategy, USP considered the global public health landscape and sought to identify problems such as vulnerabilities in the global drug supply chain, increasing digitization of healthcare, advances in manufacturing and analytics technologies, and the introduction of new classifications and new drugs.
the next five-year strategy will focus on standards, advocacy and capacity-building, as well as supporting the driving force of the strategy.
details about the strategy to members of the USP employee leadership team. jaap Venema, executive vice president and chief scientific officer of
, explains that USP's goal is to be an authoritative source of quality standards, noting that the meaning of "authority" varies in every area, such as small molecules, biologics, dietary supplements, or foods.
highlighted the explosive growth of new science in the fields of complex drugs, biologics, digital therapy and precision medicine.
commented that USP's goal is to increase the relevance of its standards to maximize impact and help ensure high-quality products in these areas.
, senior vice president of global external affairs at The World Bank, said USP's ambition in advocacy is to become a global institutional leader in improving the quality of medicines.
framework, he provided several specific examples, including concerns about the quality of USP antimicrobials, noting that evidence developed by the USP Quality Institute revealed a link between nonconforming drugs and antimicrobial resistance.
, senior vice president of global health at the U.S. Pharmacopoeia, said of capacity-building that the U.S. Pharmacopeia will become a leading service provider to improve patient health by promoting the availability of high-quality medicines.
She cites an improvement in oxytocin in collaboration with Nigerian regulators, combining USP standards, capacity-building and advocacy to help overcome high temperatures in the supply chain and improve the availability of quality products.
's 15 priority resolution resolutions over the next five years are a unique aspect of the USP governance process, providing a formal and institutionalized channel for USP Convention member organizations and other stakeholders to inform and guide USP's strategic direction at the beginning of each five-year cycle.
resolution is developed by the USP Convention Council and considered by the USP Board of Directors and the Committee of Experts.
then the resolution is reflected in the organization's policy and operational agenda and used to adjust and plan.
meeting of the USP Convention, representatives of member organizations considered 15 proposed resolutions and made a total of 230 comments on them before and during public hearings.
include concerns about resolutions, some of which are considered to go beyond the core work of the USP or require too many resources to implement.
the USP Convention Committee responded to those comments and amended six resolutions.
resolution focused on supporting medical innovations in digital, biological and advanced therapeutic areas, and highlighted the contribution that USP could make to the development and distribution of standards for cannabis products.
Final resolution on USP priorities for 2020-2025 is as follows: Resolution 1: Working with the FDA and other stakeholders on health priorities USP will continue to fulfill its commitment to leverage USP's capabilities to help promote patient safety, public health, innovation and access by identifying common priorities based on scientific principles and by identifying common priorities.
2: Efficiency in standard-setting and revision USP will actively evaluate and improve the process of developing and updating monographs and other standards to maintain and continuously optimize the impact of these standards.
, USP will consider the views and opinions of FDA, industry, and other stakeholders on process changes.
This work focuses on exploring new ways to efficiently share information, including information critical to standard-setting, as well as information needed to evaluate appropriate analytical methods and quality standards, and to integrate appropriate scientific and production progress into usP standards.
resolution 3: Quality standards USP will be an authoritative source of public quality standards and a recognized scientific leader to help protect patient and consumer safety and meet the needs of regulators, policy makers, healthcare practitioners and industries in an evolving global regulatory environment.
, USP will strive to identify emerging trends, be consistent with analytics, manufacturing, and other technological advances, and develop innovative and flexible ways to meet current and future needs of industry, regulators, healthcare practitioners, consumers, and patients.
Resolution 4: UsP for Access to Biological Products will develop standards and other solutions to support innovations in the efficient development and manufacture of high-quality biologics and advanced therapies and increase access to these medicines.
5: Innovative USP will explore the development of quality standards and other solutions used to help stakeholders safeguard the quality of these promising healthcare innovations that meet patient and public health needs.
Resolution 6: Standard Digital Conversion USP will create interoperable core digital solutions that leverage USP's data and standards to improve public health through global access to high-quality medicines.
7: Education and training for industry and healthcare professionals will build and strengthen the basic capabilities required by industry and healthcare practitioners to use the USP standard through efficient, effective and measurable training and education programs.
8: Strengthen the regulatory system USP will work with global regulators and other partners to strengthen the regulatory system.
Resolution 9: Dosing USP will continue to work with stakeholders on standards to help ensure the quality of the preparation.
will develop new and revised dosing standards, including expiration dates, based on data, scientific evidence, and advice from recognized health professionals and state and federal regulators.
resolution 10: Marijuana USP will use its scientific expertise and appeal to work with stakeholders to develop purposeful scientific resources and solutions that will help address quality-related issues and support more scientific research into cannabis, cannabis derivatives and cannabis-related compounds.
Resolution 11: Pharmacopeia Cooperation and Convergence USP will lead the promotion of convergence among pharmacopeia around strong science-based standards.
USP will focus on convergence of standards that will have the greatest impact on global access to high-quality medicines.
12: Evidence that guides policy generates and disseminates evidence that makes informed choices about investment regulation and quality systems, and reforms regulatory paradigms to improve quality, patient safety, and public health.
Resolution 13: Alliance Building USP will lead and promote stakeholder quality campaigns to advance public health and patient safety.
Resolution 14: Culture of Excellence USP will set an industry for operational excellence, continuous improvement, stakeholder response and transparency.
15: Impact expansion USP will expand public health impact by attracting more people to more regions through USP standards, capacity-building, and advocacy.