-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
17 January 2021 // -- Teva Pharmaceutical Information Consulting (Shanghai) Co., Ltd. recently announced that its innovative drug Antany ® Alustedo, common name: deutetrabenazine, is available in China for the treatment of dance diseases associated with Huntington's disease (HD) and adult delayed movement disorders (TDs).
in China, ® was approved by the State Drug Administration in May 2020.
it is worth noting that Austedo is the world®'s first approved drug, while China is the second country in the world to approve Austedo after the United States.
As part of the rapid review process, NMPA previously included Austedo on the List of Clinically Urgently Needed New Drugs (First Batch) and gave priority to review, eventually completing the approval process within four months for the benefit of Chinese patients.
Antatim® uses niobium technology that gives a good pharmacogenetic curve to the active ingredient, allowing for a reduction in the frequency of dosage, while demonstrating effectiveness and acceptable safety and tolerance for patients with Huntington's (HD) dance disease and adult delayed movement disorders.
In addition, on 28 December 2020, Antany ® was officially included in the National List of Essential Medical Insurance, Industrial Injury Insurance and Maternity Insurance Drugs (2020), which will greatly improve the burden of treatment for patients and improve access to innovative therapies.
(HD) is a rare and deadly neurodegenerative disease with an overall prevalence rate of 0.40 per 100,000 people in Asia, with an average age of 40 years.
(unconscious, random, and sudden twisting and/or rotational movements) is one of the most significant physical manifestations of the disease, appearing in about 90% of patients.
Wu Zhiying, vice president of the Second Hospital affiliated with Zhejiang University School of Medicine and director of the Center for Neurology Research, said: "Huntington's disease has no special drugs, and the current treatment is mainly empirical.
Antaltan® is currently one of the few drugs in the world that is considered to control the symptoms of Huntington's (HD) dance disease, entering China will enrich the clinical treatment options for doctors, this time included in the health insurance is also a public expectation, which is a testament to the importance that the state attaches to the group of patients with rare diseases.
" delayed movement disorder (TD) is a debilitating movement disorder characterized by repeated and uncontrollable movements of the tongue, lips, face, body and limbs.
TD has a 33.7 percent prevalence rate among Chinese schizophrenic patients who have been treated with antipsychotic drugs for a long time, possibly caused by certain drugs used to treat mental health conditions, meaning that one-third of people with schizophrenia who use these drugs may have TD.
the disease not only affects the patient's treatment compliance, but also affects the patient's quality of life and their social function.
there is currently no treatment for TD in China.
is the® first drug approved in China to treat TD, significantly reducing abnormal involuntary movement and good tolerance in patients with TD, giving patients hope of improving quality of life and social function.
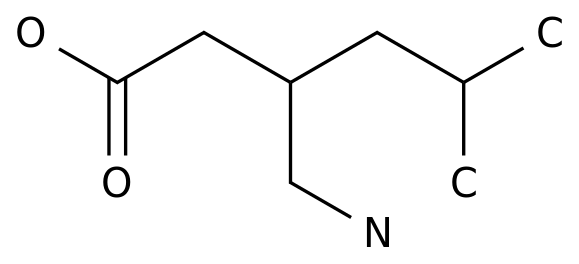
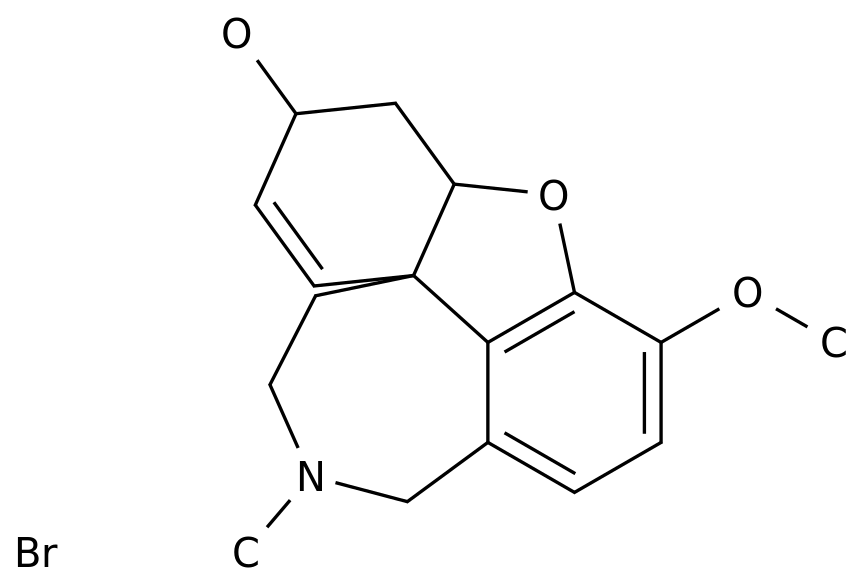
Austedo: The active ingredient in the world's first antidote drug, Austedo ®, is doutetrabenazine, a small molecule oral inhibitor that targets the vesicle monoamine transporter 2 (VMAT2), which regulates levels of dopamine, 5-hydroxysamine, epinephrine, denephrine and other chemicals in the brain.
deutetrabenazine is a drug that has been marketed as the huntington's disease treatment drug tetrabenazine.
, pharmacodynamic characteristics are improved and half-life is significantly extended, allowing for lower therapeutic doses.
Austedo is the world's first approved drug.
the United States, Austin was approved by the FDA in April 2017 for the treatment of dance diseases associated with Huntington's disease.
August 2017, the FDA approved Austredo for a new adaptive disorder for the treatment of delayed movement disorders in adults.
: The pioneering technology in the field of xenonization, Xenon (D) elements are abundant in nature and form stable molecular bonds with other elements.
in an adult, the average D content is about 2g.
although D and hydrogen (H) are basically the same in size and shape of the atom, there is also a fundamental difference between D and H, i.e. D contains an additional nucleus.
result, the chemical bonds formed by D and carbon (C) are more stable than between H and C.
, D-C chemical bonds are 6-9 times more stable than H-C chemical bonds, which has a very important impact on drug development, as drug metabolism often involves the break of H-C chemical bonds.
traditional drug discovery methods, it takes a long time and has a high failure rate.
chemical methods, usually based on drugs already on the market, are more efficient and less costly to develop.
The use of radon (thorium replacement) can enhance some of the properties of the drug: because D and C can form more stable chemical bonds, Dization in some cases can change the metabolism of the drug, including improving metabolic stability, reducing the formation of toxic metabolites, increasing the formation of the desired active metabolites, or a combination of these effects.
Compared to the corresponding non-cyclytic similars, the half-life of the compound in the body is prolonged and system exposure increases, these properties may bring therapeutic benefits such as increased safety, effectiveness, toerability and convenience.
, radon compounds are expected to retain bioaturative effects and selectivity similar to their hydrogenated similarity.
effects of radon replacement on metabolic properties are highly dependent on the specific molecular position of D to replace H.
, the metabolic effects, if any, of radon replacement are unpredictable, even in compounds with similar chemical structures.
currently, a number of pharmaceutical companies are developing currently on-market drugs of radon.
, for example, Concert has developed a new product, CTP-543, using jak1/JAK2 inhibitor ruxolitinib using argon chemistry, with strong results in the treatment of baldness.
ruxolitinib has been approved for sale under the brand name Jakafi in the United States for the treatment of a variety of blood diseases.
chemical modification of ruxolitinib can change its human pharmacological dynamics, thereby enhancing its use as a baldness treatment.
() Original origin: Teva, <!--/ewebeditor:page->