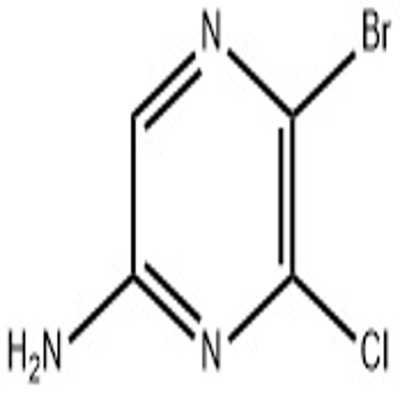
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
With the implementation of the Drug Administration Law in December 2019, a number of supporting regulations and policies for 2020 have come into effect, and the regulations implemented on July 1, 2020 include the Measures for the Supervision and Administration of Drug Production, the Measures for the Administration of Drug Registration, the Appendix to Biological Products, the Code of Quality Management of Drug Clinical Trials, the Classification and Declaration of Biological Products Registration and DeclarationInformation Requirements" Chemical Registration Classification and Declaration Data Requirements, Non-Organizational Emission Control Standards for Volatile Organics, Technical Requirements for Ozone Generators for Water Treatment, Code of Conduct for Live Webcast marketing, 12 "Medical Device Cleaning Agents," "Independent Software for Medical Device Quality Management Practices, and Interim Tax Rate Adjustment for Imports of Medical Devices 2020."on March 30, 2020, the official website of the State Administration of Market Supervision and Administration published the Measures for the Supervision and Administration of Drug Production, which will be officially implemented on July 1, 2020As the core supporting regulations in the field of drug supervision, the two measures fully implement the latest requirements of the Vaccine Administration Law and the Drug Administration Law, fully reflect the results of the reform of the national drug review and approval system in recent years, make a new system design for drug registration management and drug production supervision, make a new system design, more clear problem orientation, more scientific supervision and management, and comprehensively improve the rule of law, standardization level of drug registration and drug production, and ensure the effective and effective quality of drug productiontwo methods to encourage drug research and development innovation, the system solves the actual problems encountered in the process of declaration of innovative drugs, and fills some of the gaps in the previous systemThe simultaneous revision and publication of the two measures reflect the coordination between drug registration and post-marketing supervisionthe two measures further clarify the national and provincial drug regulatory departments in the drug registration management and day-to-day supervision of the division of power and supervision and inspection requirements, to ensure that drug registration acceptance, review, inspection and inspection and other links effectively linked, formed a drug research and development, registration declaration, on-site verification, after-market supervision of the whole process of supervision chain, to solve the drug registration and regulatory licensing matters over the years in mutual dependence, lack of proceduresThe newly revised Measures for the Supervision and Administration of Drug Production clearly state that on-site verification of drug registration and compliance with the quality management norms of pharmaceutical production before listing can be carried out simultaneously, and a number of licensing problems can be resolved through one inspectiontwo approaches also further clarify the corporate responsibilityThe two methods follow the scientific supervision concept, optimize the review and approval process, fully reflect the "management" reform requirementstwo methods to encourage drug research and development innovation, the system solves the actual problems encountered in the process of declaration of innovative drugs, and fills some of the gaps in the previous systemThe simultaneous revision and publication of the two measures reflect the coordination between drug registration and post-marketing supervisionthe two measures further clarify the national and provincial drug regulatory departments in the drug registration management and day-to-day supervision of the division of power and supervision and inspection requirements, to ensure that drug registration acceptance, review, inspection and inspection and other links effectively linked, formed a drug research and development, registration declaration, on-site verification, after-market supervision of the whole process of supervision chain, to solve the drug registration and regulatory licensing matters over the years in mutual dependence, lack of proceduresThe newly revised Measures for the Supervision and Administration of Drug Production clearly state that on-site verification of drug registration and compliance with the quality management norms of pharmaceutical production before listing can be carried out simultaneously, and a number of licensing problems can be resolved through one inspectiontwo approaches also further clarify the corporate responsibilityThe two methods follow the scientific supervision concept, optimize the review and approval process, fully reflect the "management" reform requirementsOn the day of the official publication of the Measures, theDrug Administration issued the "Announcement on the Implementation of the New Amendment measures for the Supervision and Administration of Drug Production", making it clear that the application for a drug production license for an enterprise and the commissioning of pharmaceutical production shall be connected with the old version of the Measures in accordance with the following methods:from July 1, 2020, the applicant engaged in the production activities of preparations, raw materials and Chinese medicine tablets shall apply for the relevant provisions of the drug productionThe existing Drug Production License will remain in effect for the duration of the periodwho has obtained the Drug Production License for the drug market license holder (hereinafter referred to as "the "holder") entrusts the production of the preparation, in accordance with the provisions of Article 16 of the Measures on Production concerning the change of the production address or the scope of productionIf the original batch of drug entrusted to produce, it shall continue to be valid for the period of validityIf theholder entrusts the production of preparations, it shall sign a entrustment agreement and quality agreement with a qualified pharmaceutical production enterprise, and the contents of the entrustment agreement and quality agreement shall comply with the relevant laws and regulationsIf the holder of theis approved for listing in the form of entrusted production during the pilot period until the implementation of the newly revised Measures for the Administration of Drug Registration, the holder shall apply to the local provincial drug regulatory department for a drug production license by July 1, 2020, having obtained a Drug Production License in accordance with the law before July 1, 2020, and its workshop or production line has not carried out gmp compliance checks, gmp compliance checks shall be carried out in accordance with the provisions of the Production MeasuresOn June 17,, the official website of the State Drug Administration issued the "State Drug Administration's announcement on the upgrading of the drug registration-related system (No72 of 2020)"; On April 26,, the new version of the GMP Appendix Biologics, which is a supporting document of the Code of Quality Management for Pharmaceutical Production (revised in 2010), will come into effect on July 1, 2020Among them, article 59 of the appendix, enterprises using real-time data acquisition information system to record data, because the information construction needs a certain period of time, should meet the relevant requirements by July 1, 2022the new edition of "Biological Products" a total of eight chapters and 63 articles, compared with the implementation of the old version of the "biological products" appendix, compared with The implementation of March 1, 2011, added 6 articles, revised 15 articles, the amendment of the provisions mainly around the further strengthening of vaccine management, regulate vaccine production and quality management behavior On April 26, , the State Drug Administration and the State Health and Care Commission jointly released a new version of the "Quality Management Practice for Drug Clinical Trials", the new version of China's GCP will be officially implemented on July 1, 2020, which is an important step in China's drug registration into the era of globalization, but also the pharmaceutical industry chain to put forward higher normative requirements the overall framework and chapter content of the new version of the Code of Quality Management of Drug Clinical Trials have been adjusted and supplemented more substantially than the current GCP, and the overall framework structure has increased the number of words from more than 9,000 words to more than 29,000 words, and the chapters have been adjusted from the original 13 chapters, 70 articles to 8 chapters and 84 Add up to the terms clause, from the original 19 to 40, while the term and its definition are advanced to chapter two, to facilitate the reader to read and understand the content of the specification for the release of the new version of the Code, the industry generally believes that the country is the next China's drug clinical research quality to improve the new requirements This means that pharmaceutical companies in the future in the development of drugs, to further strengthen the quality management of clinical trials At the same time, for the production, sales links to do the quality of strict control June 8, the State Drug Administration on the issuance of pharmaceutical clinical trial essential document preservation guidelines (No 37, 2020), these guidelines will come into effect on July 1, 2020 Pharmaceutical Clinical Trial Seeking Documents are documents that evaluate the implementation and data quality of drug clinical trials, and are used to demonstrate that researchers, applicants and inspectors have complied with the Code of Quality Management of Drug Clinical Trials and the relevant legal and regulatory requirements for drug clinical trials during clinical trials As the basis for confirming the authenticity of clinical trial implementation and the integrity of the data collected, the necessary documents for drug clinical trials are the important contents of the applicant's inspection and drug supervision and administration departments examining clinical trials, and should meet the requirements of the necessary document management in the Code of Quality Management of Drug Clinical Trials This Guideline applies to the preservation of the necessary documents for drug clinical trials for the purpose of applying for drug registration On June 30, , the State Drug Administration issued a circular on the Registration classification and declaration of biological products (No 43 of 2020) With regard to the classification of biological products, it will be implemented from 1 July 2020 The requirements for the declaration of biological products will be in effect from 1 October 2020 By September 30, 2020, the filing of the information may be submitted as required On June 30, , the State Drug Administration issued a circular on the "Regulation and Declaration of Chemical Drugs Registration and Declaration Requirements" (No 44 of 2020) With regard to the classification of chemical registrations, it will be implemented from 1 July 2020 The requirements for the registration and declaration of chemicals will be implemented from 1 October 2020 By September 30, 2020, the filing of the information may be submitted as required May 2019, the Ministry of Ecological Environment, together with the State Administration of Market Supervision and Administration, issued the "Unorganized Emissions Control Standards for Volatile Organics" (GB37822-2019), requiring new enterprises to implement the standards from July 1, 2019 From July 1, 2020, all enterprises fully implement the national standard GB37822! Nine types of VOCs violations, will be the focus of local law enforcement VOCs mainly include 30 industries, such as pharmaceuticals standards define voCs material storage non-tissue emission control requirements, VOCs material transfer and transmission of non-tissue emission control requirements, process VOCs unorganized emission control requirements, equipment and pipeline components VOCs leak control requirements, open liquid surface VOCs unorganized emission control requirements, as well as VOCs non-tissue emission collection and treatment system requirements, enterprise plant and surrounding pollution monitoring requirements the technical requirements of ozone generators for water treatment
in September 2019, the National Standards Committee issued GB/T37894-2019 "Technical requirements for ozone generators for water treatment", which stipulates the classification of ozone generators for water treatment, model marking and specifications, structural design and materials, general regulations, requirements, test methods, inspection rules, markings, packaging, transportation and storage, etc., and will be implemented on July 1, 2020! On June 24, , the China Advertising Association issued the Code of Conduct for Webcast Marketing This is the first domestic issue of the network broadcast marketing campaign on the special norms, innovative, will play a leading role in the healthy development of the industry This Code will come into effect on July 1, 2020 the Code requires that live webcast marketing activities should be fully, truthfully and accurately disclosed the information of goods or services, and consumers' right to know and to choose should be protected in accordance with the law; the Code, the goods or services promoted by merchants shall comply with the requirements of relevant laws and regulations for the quality and safety of the use of goods, in line with the performance of use, the claim to adopt standards, promises, etc., there is no unreasonable risk of endangering the safety of persons or property merchants selling special commodities such as medicines, medical devices, health food, formula for special medical purposes, etc., they shall obtain corresponding qualifications or administrative licenses in accordance with the law December 30, 2019, the China Washing Products Industry Association issued the group standard "Medical Device Cleaners" (T/ZGXX0003-2019), which will be implemented from July 01, 2020 this standard applies to cleaning agent products used in medical devices, and defines, classifies, requirements, test methods, inspection rules, markings, packaging, transportation and storage of medical device cleaning agents July 12, 2019, the State Drug Administration issued the "Independent Software of the Medical Device Manufacturing Quality Management Code Appendix" (hereinafter referred to as the Appendix), which aims to strengthen the supervision of the production of independent software medical devices and standardize the quality management of independent software production The appendix will be in effect on 1 July 2020 Appendix applies to stand-alone software, software components are performed by reference Independent software refers to software that has one or more medical purposes and does not require medical device hardware to accomplish its intended purpose and operates on a common computing platform The Appendix follows the basic principles and general requirements of software life cycle process and network security, and is a special requirement for the quality management practice of independent software production The quality management system for the production of independent software medical devices shall comply with the requirements of the Medical Device Manufacturing Quality Management Practice and the Appendix it is understood that, in order to clarify the regulatory requirements of software medical device products, China has issued software registration technical review guidelines, network security guidelines and mobile medical device guidelines, but has not yet developed the system's software quality management system requirements Therefore, on the basis of the medical device manufacturing quality management norms, the development of independent software appendices is of great significance to ensure the quality of software the release of the "Medical Device Manufacturing Quality Management Standard Appendix Independent Software" is the fifth appendix, further complementing and perfecting the technical documentation system of medical device manufacturing quality management specification the State Council's Tariff Committee issued the "2020 provisional import tax rate adjustment program" notice, from January 1, 2020, will be adjusted import tariffs on some goods From the program, it can be seen that from July 1 to December 31, 2020, more than 20 medical device products, including endoscopes, kidney dialysis equipment (artificial kidneys), including, the import tax rate decreased to 0.7% and below The plan clearly states that the provisional import tax rate for 859 goods (excluding tariff quota shipping) will be applied to 859 goods (excluding tariff quotas) from 1 January 2020 and that the provisional import tax rate for 7 information technology products will be abolished from 1 July 2020 2 The MFN rate for information technology products listed in the Schedule to the Amendment to the Amendment to the Accession of the World Trade Organization of the People's Republic of China will be implemented as of July 1, 2020.