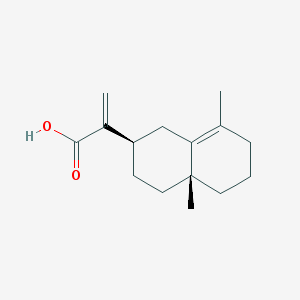
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
Recently, Yangtze River Pharmaceutical Group Shanghai Haini Pharmaceutical Co.
, Ltd.
has entered the approval stage for the application of 4 types of generic Apixaban tablets, and is expected to become the 20th domestic pharmaceutical company approved for the product
.
Apixaban is a blockbuster oral anticoagulant with global sales of more than ten billion U.
S.
dollars.
It has been included in the third batch of national procurement.
However, unsuccessful companies such as Kelun and Qingfeng still achieved good results in 2021 and are about to enter the game.
The potential of the Yangtze River can be expected
.
, Ltd.
has entered the approval stage for the application of 4 types of generic Apixaban tablets, and is expected to become the 20th domestic pharmaceutical company approved for the product
.
Apixaban is a blockbuster oral anticoagulant with global sales of more than ten billion U.
S.
dollars.
It has been included in the third batch of national procurement.
However, unsuccessful companies such as Kelun and Qingfeng still achieved good results in 2021 and are about to enter the game.
The potential of the Yangtze River can be expected
.
Figure 1: Yangtze River's products recently entered the approval stage
Source: NMPA official website
Apixaban is a highly selective and reversible factor Xa inhibitor.
It is an oral anticoagulant.
It is used in adult patients undergoing elective hip or knee replacement surgery to prevent venous thromboembolic events (VTE).
This product is taken Convenient and help improve patient compliance
.
According to data from Meinenet, the global sales of Apixaban in 2020 will exceed US$14 billion
.
It is an oral anticoagulant.
It is used in adult patients undergoing elective hip or knee replacement surgery to prevent venous thromboembolic events (VTE).
This product is taken Convenient and help improve patient compliance
.
According to data from Meinenet, the global sales of Apixaban in 2020 will exceed US$14 billion
.
In 2019, the first domestic imitation of apixaban tablets was approved.
So far, 19 domestic pharmaceutical companies have obtained apixaban tablets production approval.
If Yangtze River is successfully approved, it will become the 20th company.
Huahai Pharmaceutical, Chengdu Baidu More than 10 pharmaceutical companies such as Yu Pharmaceutical are under review.
The product is currently the most concerned anti-thrombotic drug among domestic pharmaceutical companies
.
So far, 19 domestic pharmaceutical companies have obtained apixaban tablets production approval.
If Yangtze River is successfully approved, it will become the 20th company.
Huahai Pharmaceutical, Chengdu Baidu More than 10 pharmaceutical companies such as Yu Pharmaceutical are under review.
The product is currently the most concerned anti-thrombotic drug among domestic pharmaceutical companies
.
Figure 2: The situation of companies that won the bid for apixaban tablets in China
Source: Minet.
com MID Drug Index Comprehensive Database
com MID Drug Index Comprehensive Database
The third batch of National Procurement opened bids in August 2020 and began to be implemented in the fourth quarter.
A total of 4 domestic pharmaceutical companies won the bids, all of which were approved under the new classification as over-evaluated
.
A total of 4 domestic pharmaceutical companies won the bids, all of which were approved under the new classification as over-evaluated
.
In China’s urban public hospitals, county-level public hospitals, urban community centers, and township health centers (referred to as Chinese public medical institutions), the sales of apixaban tablets in 2020 will exceed 100 million yuan.
Changzhou Hengbang Pharmaceutical, Zhengda Tian Both Qing Pharmaceutical Group and Bristol-Myers Squibb have more than 20% of the market share; in 2021H1, affected by centralized procurement, the company's ranking ushered in a major reshuffle.
CP Tianqing Pharmaceutical Group rose to TOP1, but the growth rate declined significantly, and Changzhou Hengbang Pharmaceutical took advantage of the trend It fell to TOP2, while Sichuan Kelun Pharmaceutical, which had not won the bid, rose 392% to TOP3 against the trend.
Bristol-Myers Squibb fell by 90%, leaving only 2% of the market share
.
Changzhou Hengbang Pharmaceutical, Zhengda Tian Both Qing Pharmaceutical Group and Bristol-Myers Squibb have more than 20% of the market share; in 2021H1, affected by centralized procurement, the company's ranking ushered in a major reshuffle.
CP Tianqing Pharmaceutical Group rose to TOP1, but the growth rate declined significantly, and Changzhou Hengbang Pharmaceutical took advantage of the trend It fell to TOP2, while Sichuan Kelun Pharmaceutical, which had not won the bid, rose 392% to TOP3 against the trend.
Bristol-Myers Squibb fell by 90%, leaving only 2% of the market share
.
Figure 3: 2021H1 China's public medical institutions' terminal apixaban tablets' corporate structure
Source: Mi Nei.
com, China's public medical institutions terminal competition pattern
com, China's public medical institutions terminal competition pattern
In physical pharmacies in cities in China, sales of apixaban tablets are expected to remain at the level of 40 million yuan in 2021
.
Changzhou Hengbang Pharmaceutical Co.
, Ltd.
and Chia Tai Tianqing Pharmaceutical Group, which led the market earlier, have shifted their market to the hospital side due to their centralized procurement and sales, and their sales in the retail market have declined significantly
.
The growth rates of Jiangxi Qingfeng Pharmaceutical and Sichuan Kelun Pharmaceutical reached 1213% and 100% respectively.
Bristol-Myers Squibb, which was also frustrated at the hospital side, also achieved 100% growth in the retail market
.
.
Changzhou Hengbang Pharmaceutical Co.
, Ltd.
and Chia Tai Tianqing Pharmaceutical Group, which led the market earlier, have shifted their market to the hospital side due to their centralized procurement and sales, and their sales in the retail market have declined significantly
.
The growth rates of Jiangxi Qingfeng Pharmaceutical and Sichuan Kelun Pharmaceutical reached 1213% and 100% respectively.
Bristol-Myers Squibb, which was also frustrated at the hospital side, also achieved 100% growth in the retail market
.
Figure 4: The corporate structure of apixaban tablets in 2021E Chinese urban physical pharmacies
Source: Mi Nei.
com, China's urban physical pharmacy terminal competition pattern
com, China's urban physical pharmacy terminal competition pattern
Judging from the current situation, the market demand for apixaban tablets is strong, and its inclusion in the country has triggered a wave of price reductions.
However, companies that have not won the bid or are subsequently approved still have the ability to tap the market.
As a domestic giant, Yangzijiang has a well-established channel in the company.
Under the layout, it is also expected to score good results
.
However, companies that have not won the bid or are subsequently approved still have the ability to tap the market.
As a domestic giant, Yangzijiang has a well-established channel in the company.
Under the layout, it is also expected to score good results
.
Source: NMPA official website, Mi Nei.
com database
com database
Note: Meinenet China's urban entity pharmacy terminal competition pattern database is an enlarged version of the urban entity pharmacy database that covers 293 cities and above cities across the country (excluding county and rural entity pharmacies), and continuously monitors all categories
.
The above sales are calculated based on the average retail price of the product at the terminal
.
.
The above sales are calculated based on the average retail price of the product at the terminal
.
Recently, Yangtze River Pharmaceutical Group Shanghai Haini Pharmaceutical Co.
, Ltd.
has entered the approval stage for the application of 4 types of generic Apixaban tablets, and is expected to become the 20th domestic pharmaceutical company approved for the product
.
Apixaban is a blockbuster oral anticoagulant with global sales of more than ten billion U.
S.
dollars.
It has been included in the third batch of national procurement.
However, unsuccessful companies such as Kelun and Qingfeng still achieved good results in 2021 and are about to enter the game.
The potential of the Yangtze River can be expected
.
, Ltd.
has entered the approval stage for the application of 4 types of generic Apixaban tablets, and is expected to become the 20th domestic pharmaceutical company approved for the product
.
Apixaban is a blockbuster oral anticoagulant with global sales of more than ten billion U.
S.
dollars.
It has been included in the third batch of national procurement.
However, unsuccessful companies such as Kelun and Qingfeng still achieved good results in 2021 and are about to enter the game.
The potential of the Yangtze River can be expected
.
Figure 1: Yangtze River's products recently entered the approval stage
Source: NMPA official website
Apixaban is a highly selective and reversible factor Xa inhibitor.
It is an oral anticoagulant.
It is used in adult patients undergoing elective hip or knee replacement surgery to prevent venous thromboembolic events (VTE).
This product is taken Convenient and help improve patient compliance
.
According to data from Meinenet, the global sales of Apixaban in 2020 will exceed US$14 billion
.
It is an oral anticoagulant.
It is used in adult patients undergoing elective hip or knee replacement surgery to prevent venous thromboembolic events (VTE).
This product is taken Convenient and help improve patient compliance
.
According to data from Meinenet, the global sales of Apixaban in 2020 will exceed US$14 billion
.
In 2019, the first domestic imitation of apixaban tablets was approved.
So far, 19 domestic pharmaceutical companies have obtained apixaban tablets production approval.
If Yangtze River is successfully approved, it will become the 20th company.
Huahai Pharmaceutical, Chengdu Baidu More than 10 pharmaceutical companies such as Yu Pharmaceutical are under review.
The product is currently the most concerned anti-thrombotic drug among domestic pharmaceutical companies
.
So far, 19 domestic pharmaceutical companies have obtained apixaban tablets production approval.
If Yangtze River is successfully approved, it will become the 20th company.
Huahai Pharmaceutical, Chengdu Baidu More than 10 pharmaceutical companies such as Yu Pharmaceutical are under review.
The product is currently the most concerned anti-thrombotic drug among domestic pharmaceutical companies
.
Figure 2: The situation of companies that won the bid for apixaban tablets in China
Source: Minet.
com MID Drug Index Comprehensive Database
com MID Drug Index Comprehensive Database
The third batch of National Procurement opened bids in August 2020 and began to be implemented in the fourth quarter.
A total of 4 domestic pharmaceutical companies won the bids, all of which were approved under the new classification as over-evaluated
.
A total of 4 domestic pharmaceutical companies won the bids, all of which were approved under the new classification as over-evaluated
.
In China’s urban public hospitals, county-level public hospitals, urban community centers, and township health centers (referred to as Chinese public medical institutions), the sales of apixaban tablets in 2020 will exceed 100 million yuan.
Changzhou Hengbang Pharmaceutical, Zhengda Tian Both Qing Pharmaceutical Group and Bristol-Myers Squibb have more than 20% of the market share; in 2021H1, affected by centralized procurement, the company's ranking ushered in a major reshuffle.
CP Tianqing Pharmaceutical Group rose to TOP1, but the growth rate declined significantly, and Changzhou Hengbang Pharmaceutical took advantage of the trend It fell to TOP2, while Sichuan Kelun Pharmaceutical, which had not won the bid, rose 392% to TOP3 against the trend.
Bristol-Myers Squibb fell by 90%, leaving only 2% of the market share
.
Changzhou Hengbang Pharmaceutical, Zhengda Tian Both Qing Pharmaceutical Group and Bristol-Myers Squibb have more than 20% of the market share; in 2021H1, affected by centralized procurement, the company's ranking ushered in a major reshuffle.
CP Tianqing Pharmaceutical Group rose to TOP1, but the growth rate declined significantly, and Changzhou Hengbang Pharmaceutical took advantage of the trend It fell to TOP2, while Sichuan Kelun Pharmaceutical, which had not won the bid, rose 392% to TOP3 against the trend.
Bristol-Myers Squibb fell by 90%, leaving only 2% of the market share
.
Figure 3: 2021H1 China's public medical institutions' terminal apixaban tablets' corporate structure
Source: Mi Nei.
com, China's public medical institutions terminal competition pattern
com, China's public medical institutions terminal competition pattern
In physical pharmacies in cities in China, sales of apixaban tablets are expected to remain at the level of 40 million yuan in 2021
.
Changzhou Hengbang Pharmaceutical Co.
, Ltd.
and Chia Tai Tianqing Pharmaceutical Group, which led the market earlier, have shifted their market to the hospital side due to their centralized procurement and sales, and their sales in the retail market have declined significantly
.
The growth rates of Jiangxi Qingfeng Pharmaceutical and Sichuan Kelun Pharmaceutical reached 1213% and 100% respectively.
Bristol-Myers Squibb, which was also frustrated at the hospital side, also achieved 100% growth in the retail market
.
.
Changzhou Hengbang Pharmaceutical Co.
, Ltd.
and Chia Tai Tianqing Pharmaceutical Group, which led the market earlier, have shifted their market to the hospital side due to their centralized procurement and sales, and their sales in the retail market have declined significantly
.
The growth rates of Jiangxi Qingfeng Pharmaceutical and Sichuan Kelun Pharmaceutical reached 1213% and 100% respectively.
Bristol-Myers Squibb, which was also frustrated at the hospital side, also achieved 100% growth in the retail market
.
Figure 4: The corporate structure of apixaban tablets in 2021E Chinese urban physical pharmacies
Source: Mi Nei.
com, China's urban physical pharmacy terminal competition pattern
com, China's urban physical pharmacy terminal competition pattern
Judging from the current situation, the market demand for apixaban tablets is strong, and its inclusion in the country has triggered a wave of price reductions.
However, companies that have not won the bid or are subsequently approved still have the ability to tap the market.
As a domestic giant, Yangzijiang has a well-established channel in the company.
Under the layout, it is also expected to score good results
.
However, companies that have not won the bid or are subsequently approved still have the ability to tap the market.
As a domestic giant, Yangzijiang has a well-established channel in the company.
Under the layout, it is also expected to score good results
.
Source: NMPA official website, Mi Nei.
com database
com database
Note: Meinenet China's urban entity pharmacy terminal competition pattern database is an enlarged version of the urban entity pharmacy database that covers 293 cities and above cities across the country (excluding county and rural entity pharmacies), and continuously monitors all categories
.
The above sales are calculated based on the average retail price of the product at the terminal
.
.
The above sales are calculated based on the average retail price of the product at the terminal
.
Recently, Yangtze River Pharmaceutical Group Shanghai Haini Pharmaceutical Co.
, Ltd.
has entered the approval stage for the application of 4 types of generic Apixaban tablets, and is expected to become the 20th domestic pharmaceutical company approved for the product
.
Apixaban is a blockbuster oral anticoagulant with global sales of more than ten billion U.
S.
dollars.
It has been included in the third batch of national procurement.
However, unsuccessful companies such as Kelun and Qingfeng still achieved good results in 2021 and are about to enter the game.
The potential of the Yangtze River can be expected
.
, Ltd.
has entered the approval stage for the application of 4 types of generic Apixaban tablets, and is expected to become the 20th domestic pharmaceutical company approved for the product
.
Apixaban is a blockbuster oral anticoagulant with global sales of more than ten billion U.
S.
dollars.
It has been included in the third batch of national procurement.
However, unsuccessful companies such as Kelun and Qingfeng still achieved good results in 2021 and are about to enter the game.
The potential of the Yangtze River can be expected
.
Figure 1: Yangtze River's products recently entered the approval stage
Source: NMPA official website
Apixaban is a highly selective and reversible factor Xa inhibitor.
It is an oral anticoagulant.
It is used in adult patients undergoing elective hip or knee replacement surgery to prevent venous thromboembolic events (VTE).
This product is taken Convenient and help improve patient compliance
.
According to data from Meinenet, the global sales of Apixaban in 2020 will exceed US$14 billion
.
It is an oral anticoagulant.
It is used in adult patients undergoing elective hip or knee replacement surgery to prevent venous thromboembolic events (VTE).
This product is taken Convenient and help improve patient compliance
.
According to data from Meinenet, the global sales of Apixaban in 2020 will exceed US$14 billion
.
In 2019, the first domestic imitation of apixaban tablets was approved.
So far, 19 domestic pharmaceutical companies have obtained apixaban tablets production approval.
If Yangtze River is successfully approved, it will become the 20th company.
Huahai Pharmaceutical, Chengdu Baidu More than 10 pharmaceutical companies such as Yu Pharmaceutical are under review.
The product is currently the most concerned anti-thrombotic drug among domestic pharmaceutical companies
.
So far, 19 domestic pharmaceutical companies have obtained apixaban tablets production approval.
If Yangtze River is successfully approved, it will become the 20th company.
Huahai Pharmaceutical, Chengdu Baidu More than 10 pharmaceutical companies such as Yu Pharmaceutical are under review.
The product is currently the most concerned anti-thrombotic drug among domestic pharmaceutical companies
.
Figure 2: The situation of companies that won the bid for apixaban tablets in China
Source: Minet.
com MID Drug Index Comprehensive Database
com MID Drug Index Comprehensive Database
The third batch of National Procurement opened bids in August 2020 and began to be implemented in the fourth quarter.
A total of 4 domestic pharmaceutical companies won the bids, all of which were approved under the new classification as over-evaluated
.
A total of 4 domestic pharmaceutical companies won the bids, all of which were approved under the new classification as over-evaluated
.
In China’s urban public hospitals, county-level public hospitals, urban community centers, and township health centers (referred to as Chinese public medical institutions), the sales of apixaban tablets in 2020 will exceed 100 million yuan.
Changzhou Hengbang Pharmaceutical, Zhengda Tian Both Qing Pharmaceutical Group and Bristol-Myers Squibb have more than 20% of the market share; in 2021H1, affected by centralized procurement, the company's ranking ushered in a major reshuffle.
CP Tianqing Pharmaceutical Group rose to TOP1, but the growth rate declined significantly, and Changzhou Hengbang Pharmaceutical took advantage of the trend It fell to TOP2, while Sichuan Kelun Pharmaceutical, which had not won the bid, rose 392% to TOP3 against the trend.
Bristol-Myers Squibb fell by 90%, leaving only 2% of the market share
.
Enterprise business enterpriseChangzhou Hengbang Pharmaceutical, Zhengda Tian Both Qing Pharmaceutical Group and Bristol-Myers Squibb have more than 20% of the market share; in 2021H1, affected by centralized procurement, the company's ranking ushered in a major reshuffle.
CP Tianqing Pharmaceutical Group rose to TOP1, but the growth rate declined significantly, and Changzhou Hengbang Pharmaceutical took advantage of the trend It fell to TOP2, while Sichuan Kelun Pharmaceutical, which had not won the bid, rose 392% to TOP3 against the trend.
Bristol-Myers Squibb fell by 90%, leaving only 2% of the market share
.
Figure 3: 2021H1 China's public medical institutions' terminal apixaban tablets' corporate structure
Source: Mi Nei.
com, China's public medical institutions terminal competition pattern
com, China's public medical institutions terminal competition pattern
In physical pharmacies in cities in China, sales of apixaban tablets are expected to remain at the level of 40 million yuan in 2021
.
Changzhou Hengbang Pharmaceutical Co.
, Ltd.
and Chia Tai Tianqing Pharmaceutical Group, which led the market earlier, have shifted their market to the hospital side due to their centralized procurement and sales, and their sales in the retail market have declined significantly
.
The growth rates of Jiangxi Qingfeng Pharmaceutical and Sichuan Kelun Pharmaceutical reached 1213% and 100% respectively.
Bristol-Myers Squibb, which was also frustrated at the hospital side, also achieved 100% growth in the retail market
.
Hospital hospital hospital.
Changzhou Hengbang Pharmaceutical Co.
, Ltd.
and Chia Tai Tianqing Pharmaceutical Group, which led the market earlier, have shifted their market to the hospital side due to their centralized procurement and sales, and their sales in the retail market have declined significantly
.
The growth rates of Jiangxi Qingfeng Pharmaceutical and Sichuan Kelun Pharmaceutical reached 1213% and 100% respectively.
Bristol-Myers Squibb, which was also frustrated at the hospital side, also achieved 100% growth in the retail market
.
Figure 4: The corporate structure of apixaban tablets in 2021E Chinese urban physical pharmacies
Pharmacy drugstore Source: Mi Nei.
com, China's urban physical pharmacy terminal competition pattern
com, China's urban physical pharmacy terminal competition pattern
Judging from the current situation, the market demand for apixaban tablets is strong, and its inclusion in the country has triggered a wave of price reductions.
However, companies that have not won the bid or are subsequently approved still have the ability to tap the market.
As a domestic giant, Yangzijiang has a well-established channel in the company.
Under the layout, it is also expected to score good results
.
However, companies that have not won the bid or are subsequently approved still have the ability to tap the market.
As a domestic giant, Yangzijiang has a well-established channel in the company.
Under the layout, it is also expected to score good results
.
Source: NMPA official website, Mi Nei.
com database
Source: NMPA official website, Mi Nei. com database
com database
Note: Meinenet China's urban entity pharmacy terminal competition pattern database is an enlarged version of the urban entity pharmacy database that covers 293 cities and above cities across the country (excluding county and rural entity pharmacies), and continuously monitors all categories
.
The above sales are calculated based on the average retail price of the product at the terminal
.
.
The above sales are calculated based on the average retail price of the product at the terminal
.