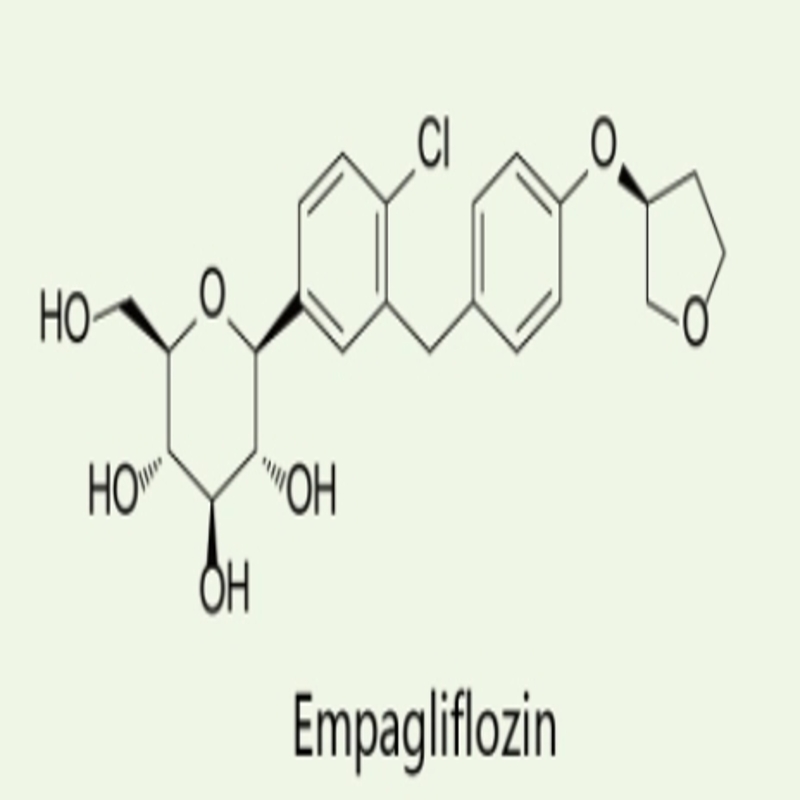
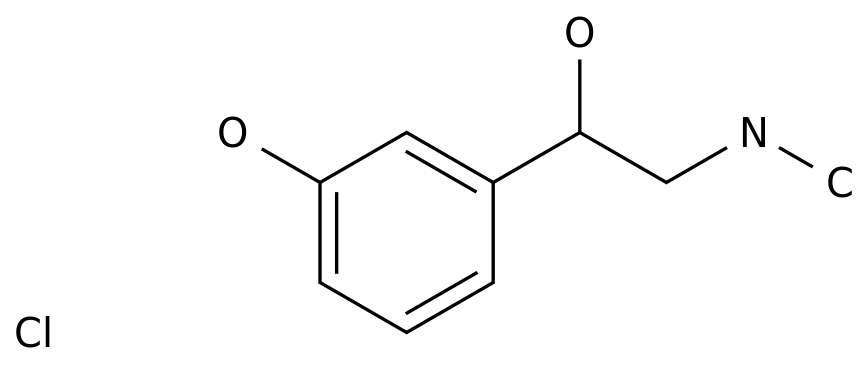
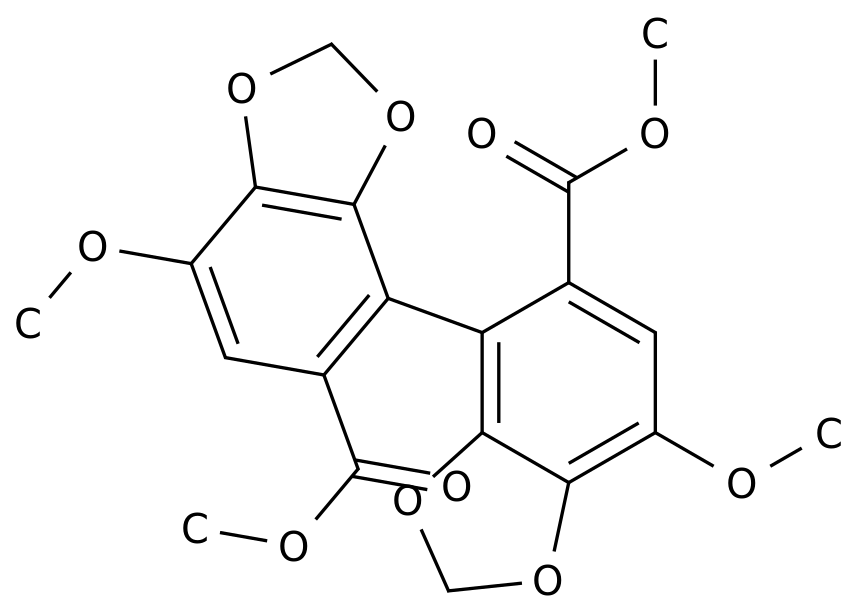
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
Yimaitong edited and arranged, please do not reprint
without authorization.
According to the National Medical Products Administration (NMPA) and related pharmaceutical companies,
of persistently decreasing estimated glomerular filtration rate (eGFR), end-stage
The approval of the indication is based on the positive results of the
in adults and
The number of patients with chronic kidney disease in China is as high as 120 million
Chronic kidney disease (CKD) is a severe condition in which patients experience a progressive decline in kidney function (marked by decreased eGFR or kidney damage or both, and persists for more than three months
).
The most common causes of chronic kidney disease are diabetes,
.
Patients with chronic kidney disease are at significantly increased
risk of complications and cardiovascular events such as heart failure and premature death.
Once a patient enters the most severe stage of chronic kidney disease, end-stage renal disease (ESKD), his kidneys are severely damaged and his kidney function progressively deteriorates, and he can only be kept alive
through dialysis or kidney transplantation.
Most people with chronic kidney disease die
from
There are currently up to 120 million patients with chronic kidney disease in
China.
39% reduction in relative risk for primary cardiorenal endpoints and 31% relative risk of all-cause mortality
The DAPA-CKD Phase III trial showed that dapagliflozin reduced the relative risk of the primary endpoint of deterioration of renal function, end-stage renal disease, cardiovascular disease, or death from cardiovascular disease by 39% compared with placebo in patients with elevated stage 2 to 4 urine
3%, p<0.
0001) compared with placebo.
。 Compared with placebo, dapagliflozin significantly reduced the relative risk of all-cause mortality by 31% in patients with chronic kidney disease (ARR=2.
1%, p=0.
0035).
The safety and tolerability of dapagliflozin are consistent
with the known safety profile of the drug.
Resources:
[1] [2]