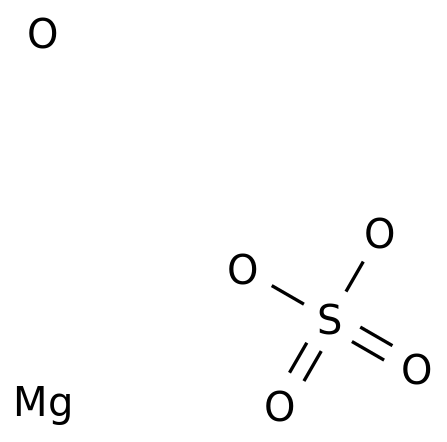
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
iNature peroxisome proliferator-activated receptor alpha (PPARα) regulates fatty acid transport and catabolism in the liver
.
However, the role of intestinal PPARα in lipid homeostasis is largely unknown
.
On April 23, 2022, Frank J.
Gonzalez of the National Institutes of Health, Qu Aijuan of Capital Medical University and Liu Weiwei of Tongji University published a joint communication online in Hepatology (IF=17) entitled "Intestinal Peroxisome Proliferator-Activated Receptor α-Fatty Acid Binding Protein 1 Axis Modulates Nonalcoholic Steatohepatitis", which found that intestinal PPARα was activated and FABP1 was upregulated in obese humans and mice fed a high-fat diet
.
Gut-specific disruption of Ppara or Fabp1 in mice fed HFD or HFCFD reduced obesity-related metabolic disturbances and NASH
.
Using luciferase reporter gene analysis and chromatin immunoprecipitation combined with fatty acid uptake analysis in primary intestinal organoids showed that intestinal PPARα targets FABP1, and FABP1 mediates the role of intestinal PPARα in regulating fatty acid uptake
.
The PPARα antagonist GW6471 ameliorates obesity and NASH, dependent on intestinal PPARα or FABP1
.
Double knockout (Ppara/Fabp1ΔIE) mice demonstrated that intestinal Ppara disruption failed to further reduce obesity and NASH in the absence of intestinal FABP1
.
GW6471 reduces human PPARA-driven intestinal fatty acid uptake and ameliorates obesity-related metabolic dysfunction in PPARA-humanized but not Ppara-null mice
.
In conclusion, this study found that intestinal PPARα signaling promotes NASH progression by regulating FABP1 to regulate dietary fatty acid uptake, which provides a compelling therapeutic target for NASH treatment
.
Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease worldwide
.
Persistent NAFLD can progress to nonalcoholic steatohepatitis (NASH), increasing the risk of end-stage liver disease such as cirrhosis and hepatocellular carcinoma
.
To date, no drug has been approved for the treatment of NASH
.
Bariatric surgery is an effective option for the treatment of morbid obesity and provides a long-term solution to NASH
.
However, surgical risks, limitations, nutritional and vitamin deficiencies, and other adverse health outcomes largely limit its use
.
Drug therapy for NASH treatment is necessary
.
Peroxisome proliferator-activated receptor alpha (PPARα) is a nuclear receptor that regulates hepatic lipid homeostasis and affects NASH progression
.
PPARα agonists such as fibrates are widely used clinically as lipid-lowering drugs to treat dyslipidemia
.
However, their use in the treatment of NASH in humans has not been approved
.
Notably, global PPARα knockdown in mice significantly enhanced NASH but prevented insulin resistance, suggesting a pleiotropic effect of PPARα
.
Understanding the tissue-specific effects of extrahepatic PPARα may guide drug discovery of PPARα modulators
.
Schematic diagram of the article (picture from Hepatology) Dietary fat is absorbed by enterocytes in the form of free fatty acids and 2-monoacylglycerols produced from dietary triglycerides (TG), while the absorbed fatty acids and monoacylglycerols are re-esterified to TG and output into the blood
.
While fatty acid-binding protein 1 (FABP1) is known to facilitate the transport of fatty acids and other hydrophobic molecules in the liver, the role of intestinal FABP1 in regulating dietary fat absorption and NASH remains unknown
.
The study found that intestinal PPARα was activated and FABP1 was upregulated in obese humans and mice fed a high-fat diet
.
Gut-specific disruption of Ppara or Fabp1 in mice fed HFD or HFCFD reduced obesity-related metabolic disturbances and NASH
.
Using luciferase reporter gene analysis and chromatin immunoprecipitation combined with fatty acid uptake analysis in primary intestinal organoids showed that intestinal PPARα targets FABP1, and FABP1 mediates the role of intestinal PPARα in regulating fatty acid uptake
.
The PPARα antagonist GW6471 ameliorates obesity and NASH, dependent on intestinal PPARα or FABP1
.
Double knockout (Ppara/Fabp1ΔIE) mice demonstrated that intestinal Ppara disruption failed to further reduce obesity and NASH in the absence of intestinal FABP1
.
GW6471 reduces human PPARA-driven intestinal fatty acid uptake and ameliorates obesity-related metabolic dysfunction in PPARA-humanized but not Ppara-null mice
.
In conclusion, this study found that intestinal PPARα signaling promotes NASH progression by regulating FABP1 to regulate dietary fatty acid uptake, which provides a compelling therapeutic target for NASH treatment
.
Reference message: https://aasldpubs.
onlinelibrary.
wiley.
com/doi/10.
1002/hep.
32538