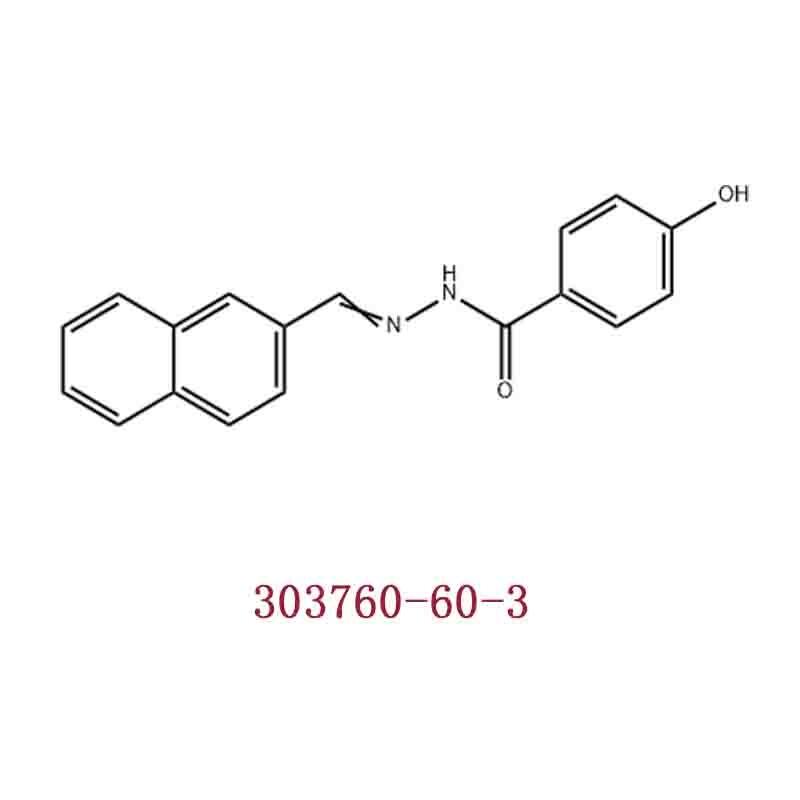
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
-
Cosmetic Ingredient
- Water Treatment Chemical
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
On August 25, news came from Xiangya Hospital of Central South University that the first patient treated by the project "Clinical Study on the Safety of Hepatocytes Using Human Embryonic Stem Cells to Induce and Differentiate Hepatocytes in the Treatment of Acute/Acute-Acute-Chronic Liver Failure" is currently living in good condition.
China is a country with a large number of liver diseases, with more than 6 million new cases of end-stage liver disease every year
After making artificial livers three times in a row, he became the first person to eat crabs
Mr.
Mr.
In May, due to the rapid progress of liver failure, he underwent artificial liver plasma exchange for three consecutive times, but with little effect
After evaluation and negotiation by the medical expert group and research team, as well as fully informed communication with patients and their families, Mr.
The effect is obvious, Mr.
Huang Yan introduced that liver function and coagulation function are the most critical data to clinically determine the progression of liver failure in this patient
More than a month after receiving stem cell therapy, Mr.
Now, Mr.
"I'm a person who came back from the brink of death, so I cherish the present more
Therapeutic Mechanism: "Universal Cells" Induce Differentiation of Liver Cells
Professor Huang Yan, director of the Department of Infectious Diseases at Xiangya Hospital of Central South University, who is in charge of the clinical study, introduced that liver failure is a serious liver damage caused by a variety of factors, with a short course of disease and acute onset.
Professor Lu Guangxiu, director of the National Engineering Research Center for Human Stem Cells, introduced that the cell preparation used to treat Mr.
Previous preclinical efficacy studies have shown that liver cell preparations can significantly improve the survival rate of animal models of liver failure - compared with the control group's survival rate of only 6.
This research is expected to be used in clinical practice as soon as possible
According to reports, on April 13, 2022, the project "Clinical study on the safety of hepatocytes induced and differentiated by human embryonic stem cells in the treatment of acute/acute-on-chronic liver failure", co-organized by the Department of Infectious Diseases of Xiangya Hospital and the National Engineering Center for Human Stem Cells, was announced.
Huang Yan introduced that the project has been reported to the National Health and Medical Commission for clinical research registration since June 2019.
The hepatocyte preparations used in the project come from the National Engineering Research Center for Human Stem Cells.
Prof.
Lingo, deputy director of the center, introduced that the hepatocyte preparations used in the project were produced in accordance with the requirements of the "Good Manufacturing Practice (GMP)", and the product quality has passed the The China National Institute for Food and Drug Control has verified that it has the function of mature liver cells, and has completed various tests such as preparation technology, cell biological identification, and characteristic analysis, which can replace human liver cells
.
At present, there are no embryonic stem cell-related cell preparations on the market in the world
.
The National Engineering Research Center for Human Stem Cells will speed up the application process of new drugs for the treatment of liver failure with human embryonic stem cell-derived hepatocytes
.