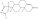-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
Lysine has a high abundance (5.
Usually, the ε-amino group of lysine is easily protonated under physiological conditions, so it is very challenging to covalently modify lysine residues of target proteins under physiological conditions
Recently, Associate Researcher Li Bo and Zhu Weiliang of Shanghai Institute of Materia Medica, Chinese Academy of Sciences, together with Professor Ge Guangbo of Shanghai University of Traditional Chinese Medicine, designed and developed a new type of water-soluble primary amino group based on the method of primary amino group modification in n-butanol solution previously developed by the team [1].
This study shows that this novel water-soluble oxazolinium compound is capable of selectively modifying the lysine residues of the backbones of peptidic drugs such as pramorelin derivatives, octreotide and semaglutide, as well as bovine serum albumin K350 of SARS-CoV-2 and two highly conserved lysine residues (K5 and K61) of SARS-CoV-2 3CLpro, and SARS-CoV-2 3CLpro was inactivated by covalent modification of lysine
At the cellular level, the oxazolinium can also be used to map lysine-modified proteins in living cells, and fluorescent molecules with oxazolinium warheads can image subcellular organelles such as lysosomes
In addition, modification experiments with mixtures of eight different amino acids and different peptides with potentially reactive residues showed that the novel water-soluble oxazolinium exhibits good selectivity for the lysine quinolinylation of peptides and proteins, and is highly selective for various functional groups.
Since the quinoline ring of oxazolinium can be further modified to obtain various derivatives, it is expected to play an important role in the research of peptides and proteins for specific purposes and protein lysine modification at the level of living cells
In conclusion, a new type of water-soluble oxazolinium developed in this study can chemically and site-selectively modify lysine in peptides, proteins and living cell proteins, and has potential applications in chemical biology and innovative drug research
The first authors of the paper are doctoral student Sun Haiguo and master student Xi Mengyu of Shanghai Institute of Materia Medica, and doctoral student Jin Qiang of Shanghai University of Traditional Chinese Medicine.
Full text link
https://doi.
The development of the above water-soluble "oxazolinium" is based on the team's continuous research work on berberine structure optimization for more than ten years
Initially, seven-membered berberine compounds with improved antitumor activity and physicochemical properties were designed and synthesized based on the structure of berberine [3,4], and the bioavailability was greatly improved compared with that of berberine (from F < 1% to F =21.
On the one hand, this compound can exert anti-multiple myeloma activity in vitro and in vivo by dual inhibiting the activity of mTORC1/2 [6], and can also be used as a skeleton of medicinal advantages to design and synthesize quaternary ammonium salt type hCES2A with auxiliary anti-tumor effect.
On the other hand, based on DCZ0358, the regioselective chemical coupling reaction of its oxazoline quaternary ammonium salt core skeleton with heteroatom (oxygen, nitrogen, sulfur) nucleophiles was studied for the first time [8], and the following year after the work was published Namely, cited by the Journal of Chemical Reviews as an example of the "cation-π" interaction controlling the regioselectivity of chemical reactions (Chem Rev.
The team also designed and synthesized a new class of compounds with anti-inflammatory activity in vitro and in vivo, and found that only the compounds in the R chiral configuration have anti-inflammatory activity and the activity of selectively binding to estrogen receptor β[9] ]
As a result, through continuous structural optimization, the selective modification of amino acid derivatives and primary amino groups of peptides in n-butanol solvent has been gradually realized [1], and the ligation of peptides, proteins and living cell proteins can currently be achieved in physiological environments.