-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
!--, 2020 // -- Novartis recently released new data for the LNP023 Treatment Of PNH Phase II Clinical Study (NCT03439839) at the 2020 Annual Meeting of the European Society for Blood and Bone Marrow Transplantation (EBMT).
LNP023 is a pioneering (first-in-class) oral, powerful, selective, complement factor B (FB) inhibitor.
PNH is a rare life-threatening blood disease characterized by supplement-driven hemolysis, thrombosis and impaired bone marrow function, leading to debilitating symptoms that can seriously affect the quality of life of patients. The results, published at the
meeting, showed that LNP023, as an additional therapy for Soliris, significantly improved hematological response and increased hemoglobin levels in PNH patients who were still active in hemolysis, anemia, and needed infusion of red blood cells, although treated with the supplement C5 inhibitor Soliris (eculizumab).
stopped using Soliris and continued to use LNP023 as a monotherapy treatment, 7 out of 10 patients maintained hemoglobin levels, the biomarkers of disease activity did not worsen, and there were no signs or symptoms of breakthrough hemolysis. "This study shows that oral LNP023 treatment avoids blood transfusions and provides meaningful clinical benefits in PNH patients who are still anaemic and dependent on blood transfusions despite receiving standard care anti-supplement drugs," said Ospedale Moscati, lead investigator of the
study and a professor at Federico II University in Naples, Italy, and head of hematology and BMT.
data clearly show that LNP023 can control the hemolysis mechanism of the disease and has the potential to change the treatment patterns of PNH.
" Novart's head of global drug development and chief medical officer, John Tsai, said: "These Phase II positive results are promising and pave the way for further assessment of oral LNP023 as a potential single drug therapy and care standard for PNH.
we will continue to develop LNP023 in this disease while exploring its application in a range of other diseases related to the complement system.
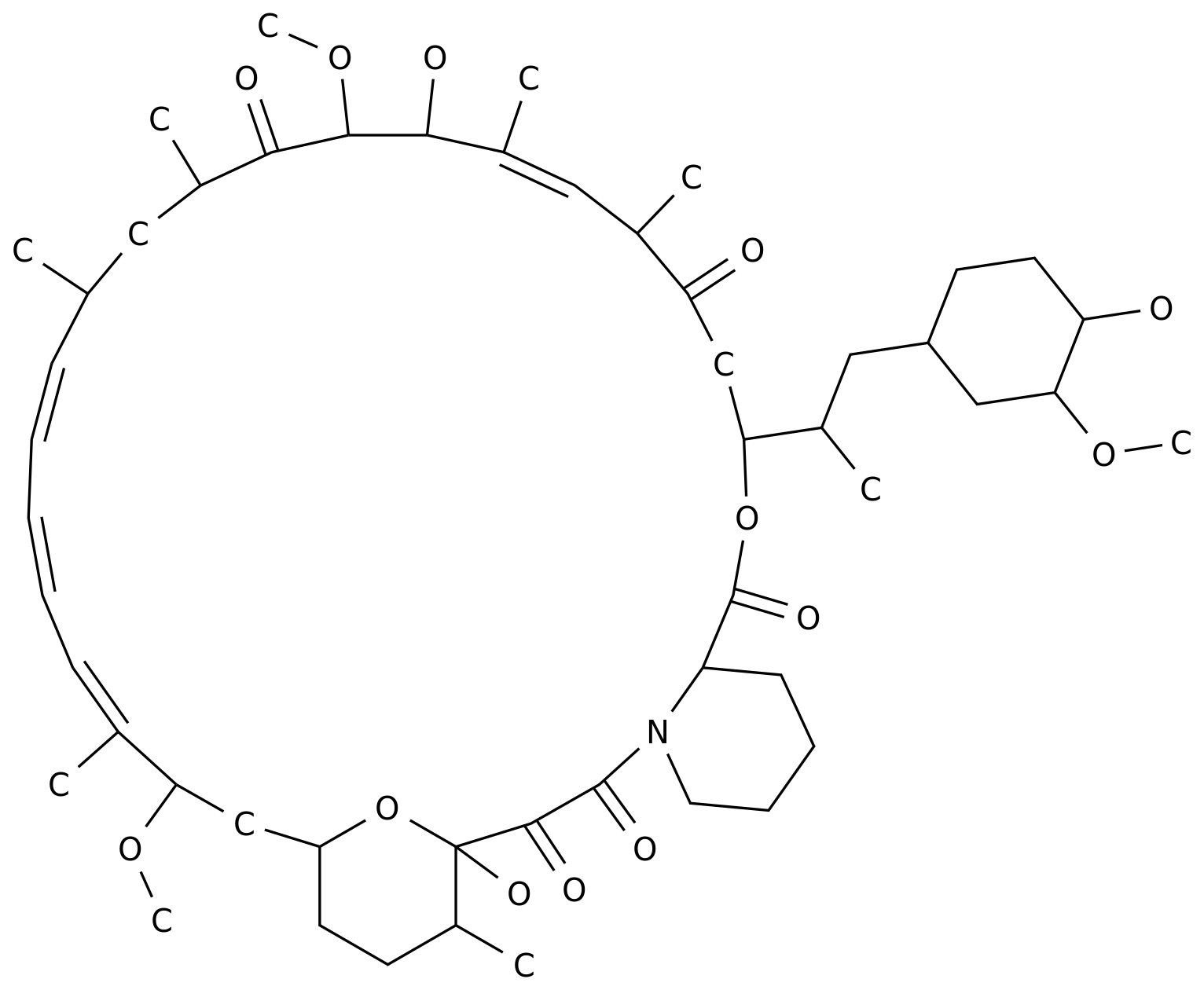
"LNP023 Chemical Structure (Photo: medchemexpress.com) LNP023 is a pioneering (first-in-class), oral, powerful, selective, complement factor B (FB) inhibitor that binds directly, reversiblely, high affinity to human complement factor B.
supplement factor B is part of the body's immune system supplement bypass.
Currently, LNP023 is clinically developed to treat PNH and a variety of kidney diseases that are affected by the supplement system and have severely unseeded needs, including IgA nephropathy, supplement 3 renal ococtrophic disease (C3G), atypical hemolytic uremia syndrome, and membrane nephropathy.
in PNH, LNP023 acts upstream of the C5 end path, preventing not only infusion of blood vessels, but also of extravascular hemolysis.
by targeting in-house pathophysiology, LNP023 may have therapeutic advantages over current standards of care.
, Novart is currently conducting another Phase II study (NCT03896152) to evaluate LNP023 as a monotherapy treatment for PNH patients who have not received anti-C5 inhibitors (anti-C5 naive) and plans to launch a Phase III study later this year.
in the United States and the European Union, LNH023 was granted the status of orphan drug (ODD) for the treatment of PNH and C3G.
Phase II study (NCT03439839) was a multi-center, open label, sequence 2 queue trial, The aim was to assess the safety, effectiveness, toerability, and pharmacodynamics/pharmacophysics of PNH patients (queue 1:n=10) who were treated with the C5 supplement inhibitor Soliris (eculizumab) but still had active hemolysis and needed infusion of red blood cells.
study was primarily aimed at assessing the effect of LNP023 on standard care (Soliris) therapy in reducing hemolysis in week 13.
the study, 13 weeks after LNP023 was treated, patients could enter a long-term extended study period that included the possibility of modifying or discontinuing Soliris treatment based on the researcher's judgment.
released at the conference, LNP023 treatment improved hematological response and biomarkers of disease activity in PNH patients who were treated with soliris but still had active hemolysis.
addition of LNP023 to Soliris therapy, patients' levels of lactic acid dehydrogenase (LDH, biomarkers of intravascular hemolysis) were significantly reduced and hemoglobin (Hb) levels significantly improved.
LNP023 increased Hb levels by 2.87 g/dL (p.001) compared to baseline values treated with Soliris alone.
addition to 2 patients, the remaining patients (80%) achieved Hb levels of 12 g/dL without blood transfusion.
all patients need to be infused with red blood cells before LNP023 treatment.
so far, seven patients (70%) have stopped using Soliris and continue to use LNP023 as a single treatment after at least six months of stable LNP023 additional treatment, according to the researchers.
importantly, hemoglobin (Hb) levels remained the same in all patients treated with LNP023 monotherapy, there was no change in biomarkers of disease activity, and there were no signs or symptoms of breakthrough hemolysis.
LNP023 also showed good safety and tolerance, with no serious treatment for related infections or thromboembolism events.
the deadline for the data provided, one participant with severe lymphocytic reduction at the start of the study was discontinued due to severe adverse events (AE) of lymphatic growth disorder.
most common adverse events are headache, insomnia, rhinitis and nasal leakage.
() !--/ewebeditor: page--!--ewebeditor:page title="--from: Novartis announces positive results from From Phase II II of LNP023 in patients with paroxysmal nocturnal hemoglobinuria (PNH) !--/ewebeditor:page