-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
11, 2020 // -- CymaBay Therapeutics is a clinical biopharmaceutical company dedicated to developing innovative therapies for liver diseases and chronic diseases with high medical needs.
recently, the company presented positive results for evaluating the treatment of primary bile bile bile bileitis (PBC) Phase III ENHANCE at the American Society for the Study of Hepatology (AASLD) liver conference.
: ENHANCE: Safety and Efficacy of Seladelpar in Patients With Primary Biliary Cholangitis.
seladelpar is a powerful, selective peroxidase proliferative activated by the subject δ (PPAR) astrogen, which has been shown to have anti-bile siltation and anti-inflammatory effects in clinical studies of the treatment of PBC.
data from the ENHANCE study confirm that seladelpar is fast, effective, safe and well-to-bear, and can quickly and significantly reduce serum alkaline phosphatase levels (ALP), have significant anti-inflammatory effects, and can rapidly and significantly reduce itching symptoms.
these results support the potential of seladelpar to be the best-in-class, breakthrough therapy drug for PBC.
, ceo and president of CymaBay Therapeutics, said: "The results of the ENHANCE study provide encouraging evidence of the anti-bile siltation, anti-inflammatory and itching effects of Seladelpar in PBC patients.
look forward to further collaboration with the medical community as we look forward to launching our RESPONSE Global Phase 3 registration study.
addition to our core focus on PBC, we will continue to explore how this new PPAR excitator promotes care and adaptation to other high unseeded needs.
"PBC" (Photo: Genfit.com) ENHANCE is a randomized, double-blind, placebo-controlled global study that included 265 PBC patients who did not respond well to UDCA (UDCA) in bears (after at least 12 months of treatment, serum alkaline phosphatase levels (ALP) ≥1.67 times normal upper limit (ULN) or poor resistance to CUDA.
study, these PBC patients were randomly assigned a placebo, seladelpar 5mg, seladelpar 10mg, or oral once a day.
the main outcome indicator of the study was the proportion of respondents, defined as patients with ALP levels of 1.67 times ULAN and a decrease of ≥15% from the baseline and normal levels of total bililin after 52 weeks of treatment.
Because of the early termination of the study and the low number of patients reaching the 52-week point in time, the main outcome indicator was modified to a three-month point in time before the database was locked out, reaching 167 out of 265 patients.
additional key analyses compared the normalization rate of the ALP with the itching burden assessed by the Numerical Assessment Scale (NRS), which was also adjusted to a 3-month point in time.
baseline examination, the average ALP levels in the placebo, 5 mg and 10 mg groups were 293, 290, 291 IU/L, respectively.
the itching NRS (0-10) score of 4 at the time of the baseline check≥ about 30% of patients had moderate to severe itching.
baseline characteristics were balanced among 3 groups and represented a high-risk PBC patient population.
results showed that seladelpar met the main composite outcome indicators and was highly statistically significant: after 3 months of treatment, 78.2% of patients in the 10mg group (n-55) and 57.1% of patients in the 5mg group (n-56) achieved the main composite results, while the placebo group (n-56) had only 12.5% (p-lt; 0.0001).
in patients treated with seladelpar, a rapid, dose-dependent decrease in ALP was observed as early as one month, with an average decrease of 38%, 30%, and 2% in the 10 mg group (n=78), 5 mg (n=78) and the placebo group (n=78).
the anti-bile siltation effect of seladelpar was further confirmed, with 27.3% of patients in the 10mg group normalizing their ALP levels at 3 months, while 0% in the placebo group (p.lt;0.0001).
at 6 months, similar patterns occurred at these endpoints, but fewer patients reached this point in the study.
in patients ≥ NRS≥4, seladelpar also showed significant, dose-dependent itching reduction after just 3 months of treatment compared to placebo.
itching NRS in the 10mg group was 3.2 points lower than the baseline, while the placebo group was on average 1.6 points lower (p.lt;0.05).
at 3 months of treatment, seladelpar also showed strong anti-inflammatory activity, with a 17% reduction in the average glutamate transaminase (ALT) in the 10 mg group and 3% in the placebo group (p.lt;05).
effects of seladelpar therapy on γ-glutamine transferase (GGT) were also significant, with a 36% decrease in the 10mg group and a 7% decrease in the placebo group (p-lt;0.0001).
total bililin remained stable in all 3 groups.
studies, seladelpar has good safety and tolerance.
adverse events in the Seladelpar group and the placebo group.
did not ≥ level 3 ALT elevated.
the > adverse events occurring in 10% of patients were itching, with the placebo group, the 5mg group and the 10mg group 12.6%, 3.4% and 11.2%, respectively.
no treatment-related adverse events, and 2 cases were discontinued due to adverse events during treatment.
Gideon Hirschfield, M.D., of the University of Toronto, said: "These data suggest that seladelpar is effective, safe and well tolerable and has the potential to be the second-line treatment of choice for PBC patients.
Given the significant unsealed medical needs of the PBC population, I am confident that researchers and patients will continue to support CymaBay's future efforts to make this breakthrough treatment available to PBC patients."
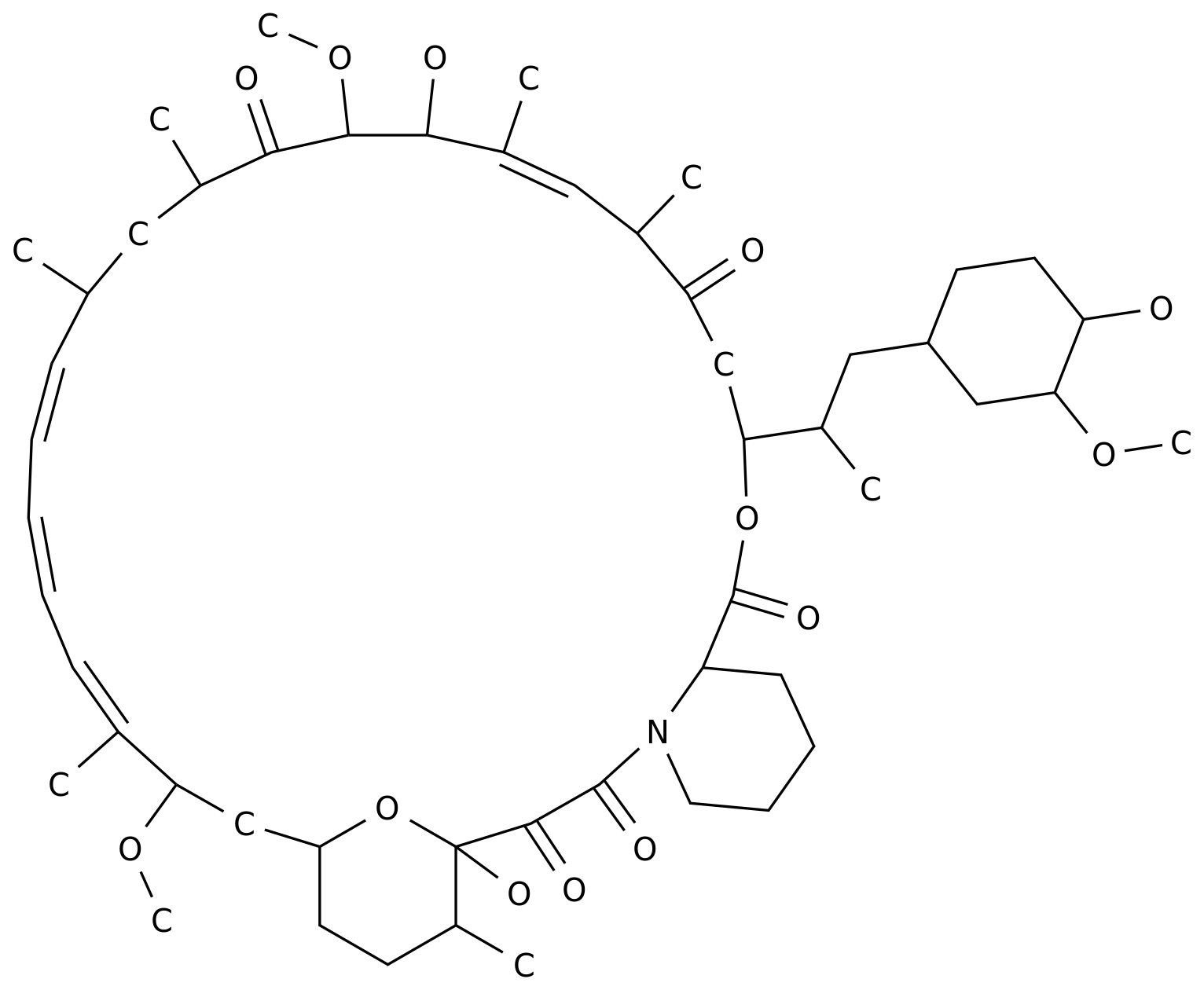
"seladelpar molecular structure (Photo: medchemexpress.cn) primary bile bile bilecholitis (PBS) is a serious, life-threatening autoimmune liver disease.
is characterized by impaired bile flow (bile siltation) and the build-up of toxic bile acid.
accompanied by inflammation and destruction of the bile tubes in the liver can develop into fibrosis, cirrhosis and liver failure.
clinical symptoms of PBC include fatigue and itching, which can be very disabling in some patients.
PBC is primarily a female disease: 1 in 1,000 women over the age of 40 has PBC.
is a powerful, selective, oral PPAR agitant that is being developed to treat liver disease PBC and non-alcoholic fatty hepatitis (NASH).
for PBC, Seladelpar has been qualified as an orphan drug by the FDA and the European Union EMA.
addition, Seladelpar Therapeutics PBC has been awarded FDA Breakthrough Drug Eligibility (BTD) and EMA Priority Drug Qualification (PRIME).
Origin: Cyma Bay Therapeutics Announcs Oral Late-Breaking Presentation of Positive Results from the ENHANCE Global Phase 3 Study Evaluating Seladelpar for Primary Biliary Cholangitis at The Liver Meeting? 2020<!--/ewebeditor:page->