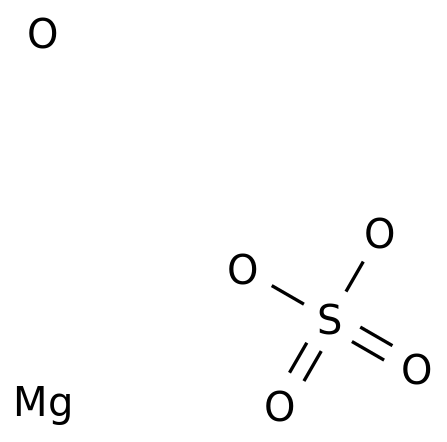
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
Non-alcoholic fatty liver disease is currently the most common liver disease.
There are nearly 2 billion patients worldwide, and the incidence is increasing rapidly
.
The prevalence rate of non-alcoholic fatty liver in China has exceeded 30%, which has become a major public health problem and a serious social medical burden
.
Fatty liver is also an important risk factor for many cardiovascular diseases, metabolic diseases, tumors, etc.
Once it develops into non-alcoholic steatohepatitis (hereinafter referred to as "fatty hepatitis"), it will significantly increase the risk of liver diseases such as cirrhosis, liver cancer, and liver failure
.
According to statistics, the probability of liver cirrhosis in patients with steatohepatitis within 10-15 years is as high as 15%-25%
.
Therefore, the clinical demand for steatohepatitis drugs is urgent, and its global market is expected to reach 35 billion U.
S.
dollars in 2030
.
In view of the huge prevalence of steatohepatitis, serious health hazards and strong clinical needs, global pharmaceutical companies such as Pfizer, Eli Lilly, Gilead, and Novo Nordisk have accelerated the development of new drugs in this field
.
At present, there are more than 800 drug trials for steatohepatitis in the world, but no drug has been approved by the U.
S.
Food and Drug Administration (FDA) to enter the clinic
.
Acetyl-Coenzyme A carboxylase (ACC) is currently the most potential therapeutic target for steatohepatitis.
Its inhibitors can significantly improve liver fatification, inflammation and fibrosis, but existing ACC inhibitors can cause serious increases in blood lipids Side effects greatly hinder its clinical application
.
The team of Professor Li Hongliang of Wuhan University has been committed to the research of major clinical problems such as fatty hepatitis for the past ten years
.
On December 15, 2021, Professor Li Hongliang’s team published two articles titled "Multiple omics study identifies an interspecies conserved driver for nonalcoholic steatohepatitis" and "A small molecule targeting ALOX12" in the top international journal Science Translational Medicine.
-ACC1 ameliorates nonalcoholic steatohepatitis in mice and macaques" cover article
.
This is the first time since the publication of "Science Translational Medicine" published two articles from the same research team back to back at the same time
.
This study revealed the core mechanism of the occurrence and development of steatohepatitis, and found that 12-lipoxygenase (ALOX12) is the key target of the steatohepatitis process.
ALOX12 can directly target ACC1, specifically and precisely regulate the ACC1 lysosomal degradation pathway
.
Based on this discovery, Professor Li Hongliang’s team has developed a new small molecule compound that can precisely target the ALOX12-ACC1 protein interaction, promote the degradation of ACC1 protein, and significantly inhibit the development of steatohepatitis
.
The ALOX12 inhibitor has a significant therapeutic effect and, more importantly, does not cause side effects such as hyperlipidemia
.
This research breaks through the problem of side effects of targeted ACC, solves the dilemma of targeted ACC in the treatment of steatohepatitis, provides an important theoretical basis for the development of ACC inhibitors, and puts forward a practical and feasible approach for the treatment of steatohepatitis by targeted ACC.
Direction
.
Figure A.
IMA-1 inhibits ALOX12 and ACC1 protein interaction structure diagram; Figure B.
IMA-1 treatment of steatohepatitis (red staining area represents the degree of liver fibrosis) "Science Translational Medicine" is an internationally renowned journal "Science" (Science) The most important sub-journal, the latest impact factor is 17.
956
.
The journal is dedicated to the study of major issues in the global translational medicine field.
The original research published in the journal is a major breakthrough in disease prevention, diagnosis or treatment, filling the gap in the integration of basic research and applied science
.
In this issue of "Science and Translational Medicine" titled "Multiple omics study identifiesan interspecies conserved driver for nonalcoholic steatohepatitis" research results, Li Hongliang team for the first time in the human, cynomolgus monkey, Bama pig, mouse and other species of systematic drawing The NASH liver transcriptome map was found, and it was found that the arachidonic acid metabolism pathway, especially ALOX12, was significantly activated in NASH and was closely related to the severity of NASH; through a series of experiments such as gene knockout and overexpression, and a panoramic systemic biology Scientific analysis, the research team fully proved that ALOX12 significantly aggravated liver fat accumulation, inflammation, fibrosis and other NASH processes
.
More importantly, Li Hongliang’s team discovered for the first time that ALOX12 can specifically bind to ACC1, inhibiting its lysosomal degradation pathway without affecting its proteasome degradation pathway; knocking out or interfering with ALOX12 can precisely regulate ACC1 protein expression and significantly improve mice And the severity of NASH in Bama pigs, without causing side effects similar to the elevated blood lipids caused by other ACC inhibitors
.
The research results of "A small molecule targeting ALOX12-ACC1 ameliorates nonalcoholic steatohepatitis in mice and macaques" published by Li Hongliang’s team back to back in the same period developed a new mechanism that specifically blocks the interaction of ALOX12-ACC1 based on the new mechanism for the development of NASH.
The new small molecule compound, named IMA-1, has fully proven its safety and effectiveness
.
Through systematic pharmacodynamic evaluation, the study found that IMA-1 can significantly inhibit the progression of NASH in mice and cynomolgus monkeys
.
More importantly, IMA-1 treatment of NASH does not cause the side effects of hyperlipidemia caused by ACC classic enzyme activity inhibitors in mice and cynomolgus monkeys
.
In the mechanism study, Li Hongliang’s team found that the degree of ACC1 inhibition is to a large extent an important reason for determining whether it induces hyperlipidemia.
Traditional ACC inhibitors almost completely replace the phosphorylation of ACC1 and ACC2, leading to ACC activity.
It is almost completely inhibited; and IMA-1 specifically blocks the interaction of ALOX12 and ACC1 and promotes its lysosomal degradation without affecting its proteasome degradation pathway
.
This research provides an important theoretical basis and reference for the development of ACC inhibitors, and proposes feasible concepts and directions for the targeted ACC treatment of NASH
.
Ten years of continuous research to solve major clinical problems of cardiovascular and metabolic diseases.
In recent years, Professor Li Hongliang’s team has focused on ALOX12 to carry out a series of studies and found that ALOX12 is the core driving factor of liver and heart ischemia-reperfusion injury, which significantly aggravates organ inflammation and damage.
; And developed small molecule inhibitors, which fully confirmed the safety and effectiveness of small molecule drugs targeting ALOX12 in the treatment of organ damage in mice, pigs, and monkeys
.
Related studies were published in Nature Medicine (Nat Med.
2018;24:73-83) and Cell Metabolism (Cell Metab.
2021;33:2059-2075.
), providing new treatments for improving the prognosis of organ ischemia-reperfusion injury Targets and strategies
.
The ALOX12 series of research work is a microcosm of the important representative research results of Professor Li Hongliang's team over the past ten years
.
Professor Li Hongliang’s team has been focusing on the pathogenesis of cardiovascular and metabolic diseases, drug treatment target research and drug development, and systematically revealed the mechanism of cardiovascular and metabolic diseases such as heart failure, ischemia-reperfusion injury, and non-alcoholic steatohepatitis.
More than 200 related research papers (more than 100 papers with an impact factor greater than 10) have been published in internationally renowned journals such as Nat Med, Sci Transl Med, Cell Metab, Circulation, Hepatology, J Hepatol, Nat Commun, PNAS, Hypertension, etc.
, which are fatty liver disease The prevention and treatment of high-incidence cardiovascular and metabolic diseases has provided important theoretical basis
.
Reference message: https://
There are nearly 2 billion patients worldwide, and the incidence is increasing rapidly
.
The prevalence rate of non-alcoholic fatty liver in China has exceeded 30%, which has become a major public health problem and a serious social medical burden
.
Fatty liver is also an important risk factor for many cardiovascular diseases, metabolic diseases, tumors, etc.
Once it develops into non-alcoholic steatohepatitis (hereinafter referred to as "fatty hepatitis"), it will significantly increase the risk of liver diseases such as cirrhosis, liver cancer, and liver failure
.
According to statistics, the probability of liver cirrhosis in patients with steatohepatitis within 10-15 years is as high as 15%-25%
.
Therefore, the clinical demand for steatohepatitis drugs is urgent, and its global market is expected to reach 35 billion U.
S.
dollars in 2030
.
In view of the huge prevalence of steatohepatitis, serious health hazards and strong clinical needs, global pharmaceutical companies such as Pfizer, Eli Lilly, Gilead, and Novo Nordisk have accelerated the development of new drugs in this field
.
At present, there are more than 800 drug trials for steatohepatitis in the world, but no drug has been approved by the U.
S.
Food and Drug Administration (FDA) to enter the clinic
.
Acetyl-Coenzyme A carboxylase (ACC) is currently the most potential therapeutic target for steatohepatitis.
Its inhibitors can significantly improve liver fatification, inflammation and fibrosis, but existing ACC inhibitors can cause serious increases in blood lipids Side effects greatly hinder its clinical application
.
The team of Professor Li Hongliang of Wuhan University has been committed to the research of major clinical problems such as fatty hepatitis for the past ten years
.
On December 15, 2021, Professor Li Hongliang’s team published two articles titled "Multiple omics study identifies an interspecies conserved driver for nonalcoholic steatohepatitis" and "A small molecule targeting ALOX12" in the top international journal Science Translational Medicine.
-ACC1 ameliorates nonalcoholic steatohepatitis in mice and macaques" cover article
.
This is the first time since the publication of "Science Translational Medicine" published two articles from the same research team back to back at the same time
.
This study revealed the core mechanism of the occurrence and development of steatohepatitis, and found that 12-lipoxygenase (ALOX12) is the key target of the steatohepatitis process.
ALOX12 can directly target ACC1, specifically and precisely regulate the ACC1 lysosomal degradation pathway
.
Based on this discovery, Professor Li Hongliang’s team has developed a new small molecule compound that can precisely target the ALOX12-ACC1 protein interaction, promote the degradation of ACC1 protein, and significantly inhibit the development of steatohepatitis
.
The ALOX12 inhibitor has a significant therapeutic effect and, more importantly, does not cause side effects such as hyperlipidemia
.
This research breaks through the problem of side effects of targeted ACC, solves the dilemma of targeted ACC in the treatment of steatohepatitis, provides an important theoretical basis for the development of ACC inhibitors, and puts forward a practical and feasible approach for the treatment of steatohepatitis by targeted ACC.
Direction
.
Figure A.
IMA-1 inhibits ALOX12 and ACC1 protein interaction structure diagram; Figure B.
IMA-1 treatment of steatohepatitis (red staining area represents the degree of liver fibrosis) "Science Translational Medicine" is an internationally renowned journal "Science" (Science) The most important sub-journal, the latest impact factor is 17.
956
.
The journal is dedicated to the study of major issues in the global translational medicine field.
The original research published in the journal is a major breakthrough in disease prevention, diagnosis or treatment, filling the gap in the integration of basic research and applied science
.
In this issue of "Science and Translational Medicine" titled "Multiple omics study identifiesan interspecies conserved driver for nonalcoholic steatohepatitis" research results, Li Hongliang team for the first time in the human, cynomolgus monkey, Bama pig, mouse and other species of systematic drawing The NASH liver transcriptome map was found, and it was found that the arachidonic acid metabolism pathway, especially ALOX12, was significantly activated in NASH and was closely related to the severity of NASH; through a series of experiments such as gene knockout and overexpression, and a panoramic systemic biology Scientific analysis, the research team fully proved that ALOX12 significantly aggravated liver fat accumulation, inflammation, fibrosis and other NASH processes
.
More importantly, Li Hongliang’s team discovered for the first time that ALOX12 can specifically bind to ACC1, inhibiting its lysosomal degradation pathway without affecting its proteasome degradation pathway; knocking out or interfering with ALOX12 can precisely regulate ACC1 protein expression and significantly improve mice And the severity of NASH in Bama pigs, without causing side effects similar to the elevated blood lipids caused by other ACC inhibitors
.
The research results of "A small molecule targeting ALOX12-ACC1 ameliorates nonalcoholic steatohepatitis in mice and macaques" published by Li Hongliang’s team back to back in the same period developed a new mechanism that specifically blocks the interaction of ALOX12-ACC1 based on the new mechanism for the development of NASH.
The new small molecule compound, named IMA-1, has fully proven its safety and effectiveness
.
Through systematic pharmacodynamic evaluation, the study found that IMA-1 can significantly inhibit the progression of NASH in mice and cynomolgus monkeys
.
More importantly, IMA-1 treatment of NASH does not cause the side effects of hyperlipidemia caused by ACC classic enzyme activity inhibitors in mice and cynomolgus monkeys
.
In the mechanism study, Li Hongliang’s team found that the degree of ACC1 inhibition is to a large extent an important reason for determining whether it induces hyperlipidemia.
Traditional ACC inhibitors almost completely replace the phosphorylation of ACC1 and ACC2, leading to ACC activity.
It is almost completely inhibited; and IMA-1 specifically blocks the interaction of ALOX12 and ACC1 and promotes its lysosomal degradation without affecting its proteasome degradation pathway
.
This research provides an important theoretical basis and reference for the development of ACC inhibitors, and proposes feasible concepts and directions for the targeted ACC treatment of NASH
.
Ten years of continuous research to solve major clinical problems of cardiovascular and metabolic diseases.
In recent years, Professor Li Hongliang’s team has focused on ALOX12 to carry out a series of studies and found that ALOX12 is the core driving factor of liver and heart ischemia-reperfusion injury, which significantly aggravates organ inflammation and damage.
; And developed small molecule inhibitors, which fully confirmed the safety and effectiveness of small molecule drugs targeting ALOX12 in the treatment of organ damage in mice, pigs, and monkeys
.
Related studies were published in Nature Medicine (Nat Med.
2018;24:73-83) and Cell Metabolism (Cell Metab.
2021;33:2059-2075.
), providing new treatments for improving the prognosis of organ ischemia-reperfusion injury Targets and strategies
.
The ALOX12 series of research work is a microcosm of the important representative research results of Professor Li Hongliang's team over the past ten years
.
Professor Li Hongliang’s team has been focusing on the pathogenesis of cardiovascular and metabolic diseases, drug treatment target research and drug development, and systematically revealed the mechanism of cardiovascular and metabolic diseases such as heart failure, ischemia-reperfusion injury, and non-alcoholic steatohepatitis.
More than 200 related research papers (more than 100 papers with an impact factor greater than 10) have been published in internationally renowned journals such as Nat Med, Sci Transl Med, Cell Metab, Circulation, Hepatology, J Hepatol, Nat Commun, PNAS, Hypertension, etc.
, which are fatty liver disease The prevention and treatment of high-incidence cardiovascular and metabolic diseases has provided important theoretical basis
.
Reference message: https://