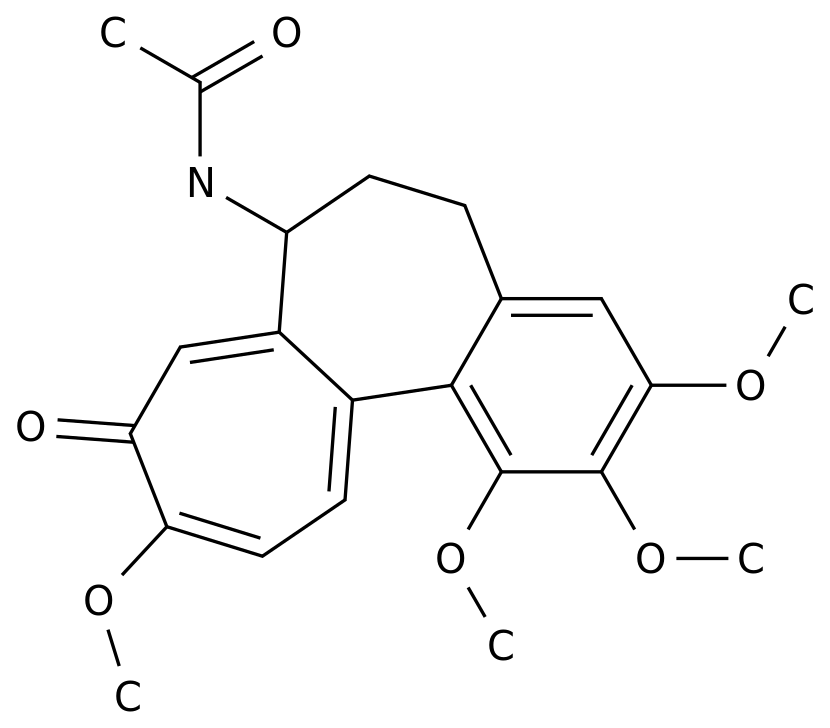
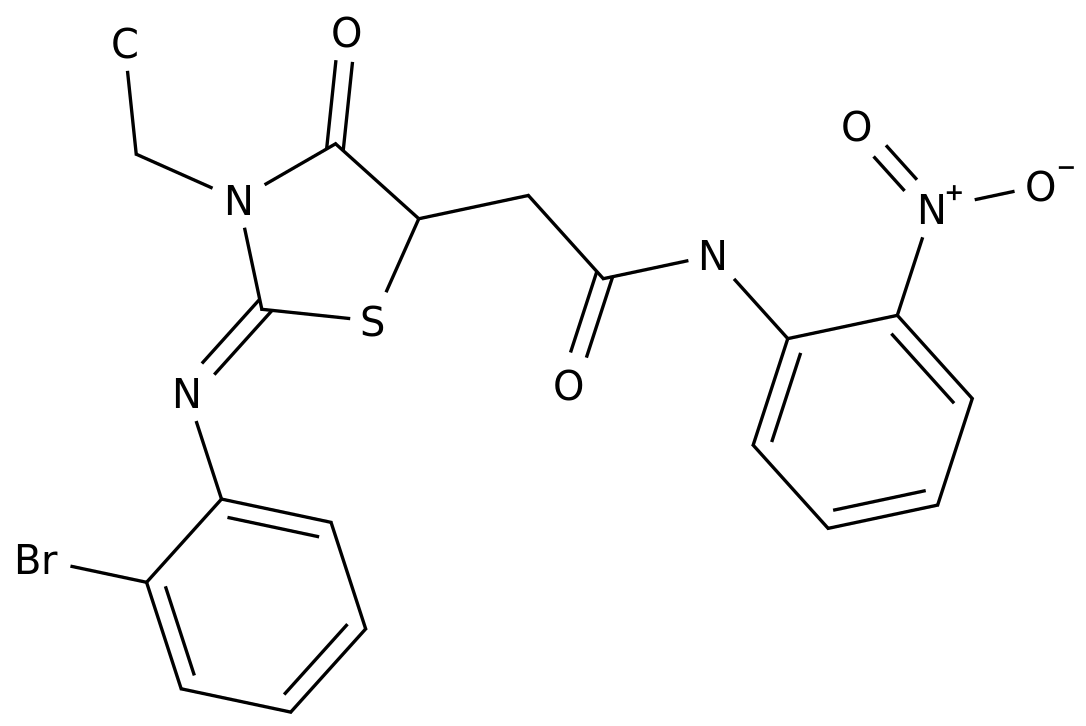
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
The "China Expert Consensus on Anti-angiogenesis Drug Treatment of Advanced Non-small Cell Lung Cancer (2022 Edition)" (hereinafter referred to as the "Consensus"), organized by the Expert Committee of Vascular Targeted Therapy of the Chinese Society of Clinical Oncology (CSCO) and the Committee on Non-small Cell Lung Cancer, and developed by the National Expert Colleagues, was successfully held
The "Consensus" conference invited members of the CSCO Vascular Targeted Therapy Expert Committee and many big names in the field of cancer prevention and treatment across the country to gather in the cloud, witnessing this important moment
Expert introduction
The Consensus and the Guidelines are closely related
CSCO has historically attached importance to the development and revision
There are links and differences between
The "Consensus" formulates scientific norms
The reporter learned that the recommended content of the "Consensus" refers to the CSCO evidence level (1-3 types of evidence) and the recommendation level (I.
Professor Li Kai further introduced that the expert group sorted out the successful experience accumulated in the use of the "Consensus" in the early stage, and paid more attention to collecting new progress in the past two years, such as the deadline of May 31, 2022, and retrieved 117 relevant English literature; Chinese 184 articles, with reference to NCCN guidelines, the Chinese Medical Association's "Guidelines for the Diagnosis and Treatment of Lung Cancer (2021 Edition)" and the "CSCO Non-small Cell Lung Cancer Guidelines"
China Experience China Program
New angiogenesis plays a key role
Professor Han Baohui pointed out in response to the inquiry that in the implementation of the "Healthy China" strategy, effectively improving the 5-year survival of lung cancer patients is an indispensable part
Professor Zhou Caicun said that since the formulation of the "Consensus", it has greatly helped the rational use of anti-angiogenic drugs in clinical practice
Widely publicize and work together
The formulation of the "Consensus" has played a guiding and normative role
Professor Zhou Caicun said that after the formulation of the "Guide" and "Consensus", CSCO promptly invited drafting experts to hold a press conference, published and published relevant books and papers, and will also organize different levels and different forms of publicity meetings
Professor Li Kai pointed out that it is born with the times and changes according to progress, and any guidelines and consensus can only have vitality
Professor Han Baohui also put forward hopes
summary
The "Consensus" conference is tightly paced and rigorous.
Looking back at the past, looking to the future